7.1: La idea 'básica' de una reacción ácido-base
- Page ID
- 2360
7.1A: The Brønsted-Lowry definition of acidity
We’ll begin our discussion of acid-base chemistry with a couple of essential definitions. The first of these definitions was proposed in 1923 by the Danish chemist Johannes Brønsted and the English chemist Thomas Lowry, and has come to be known as the Brønsted-Lowry definition of acids and bases. An acid, by the Brønsted-Lowry definition, is a species which is able to donate a proton (H+), while a base is a proton acceptor. We have already discussed in the previous chapter one of the most familiar examples of a Brønsted-Lowry acid-base reaction, between hydrochloric acid and hydroxide ion:
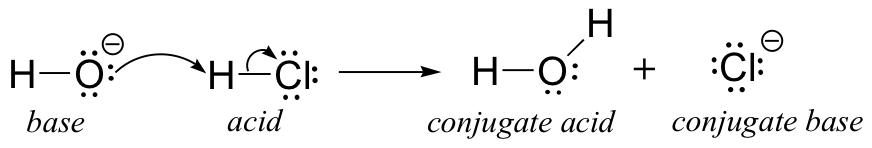
In this reaction, a proton is transferred from HCl (the acid, or proton donor) to hydroxide (the base, or proton acceptor). As we learned in the previous chapter, curved arrows depict the movement of electrons in this bond-breaking and bond-forming process.
After a Brønsted-Lowry acid donates a proton, what remains – in this case, a chloride ion – is called the conjugate base. Chloride is thus the conjugate base of hydrochloric acid. Conversely, when a Brønsted-Lowry base accepts a proton it is converted into its conjugate acid form: water is thus the conjugate acid of hydroxide.
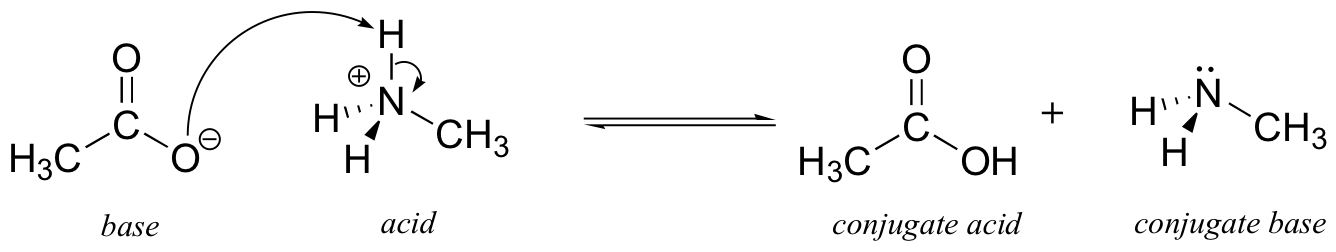
We can also talk about conjugate acid/base pairs: the two acid/base pairs involved in our first reaction are hydrochloric acid/chloride and hydroxide/water.In this next acid-base reaction, the two pairs involved are acetate/acetic acid and methyl ammonium/methylamine:
Throughout this text, we will often use the abbreviations HA and :B in order to refer in a general way to acidic and basic reactants:
![]()
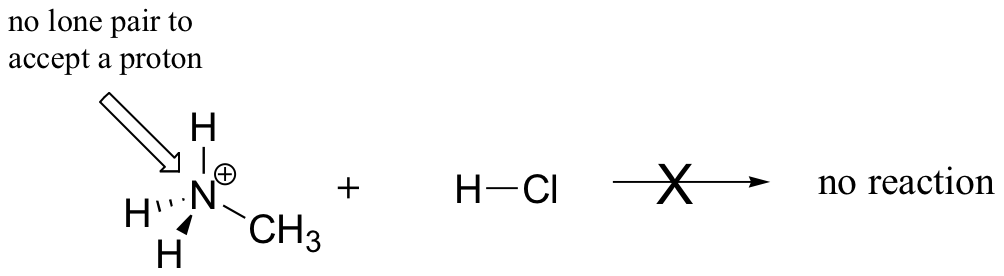
In order to act as a proton acceptor, a base must have a reactive pair of electrons. In all of the examples we shall see in this chapter, this pair of electrons is a non-bonding lone pair, usually (but not always) on an oxygen, nitrogen, sulfur, or halogen atom. When acetate acts as a base in the reaction shown above, for example, one of its oxygen lone pairs is used to form a new bond to a proton. The same can be said for an amine acting as a base. Clearly, methyl ammonium ion cannot act as a base – it does not have a reactive pair of electrons with which to accept a new bond to a proton.
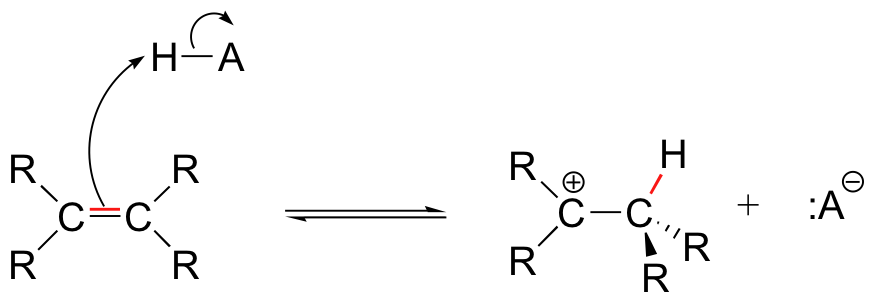
Later, in chapter 15, we will see several examples where the (relatively) reactive pair of electrons in a pi bond act in a basic fashion.
In this chapter, we will concentrate on those bases with non-bonding (lone pair) electrons.
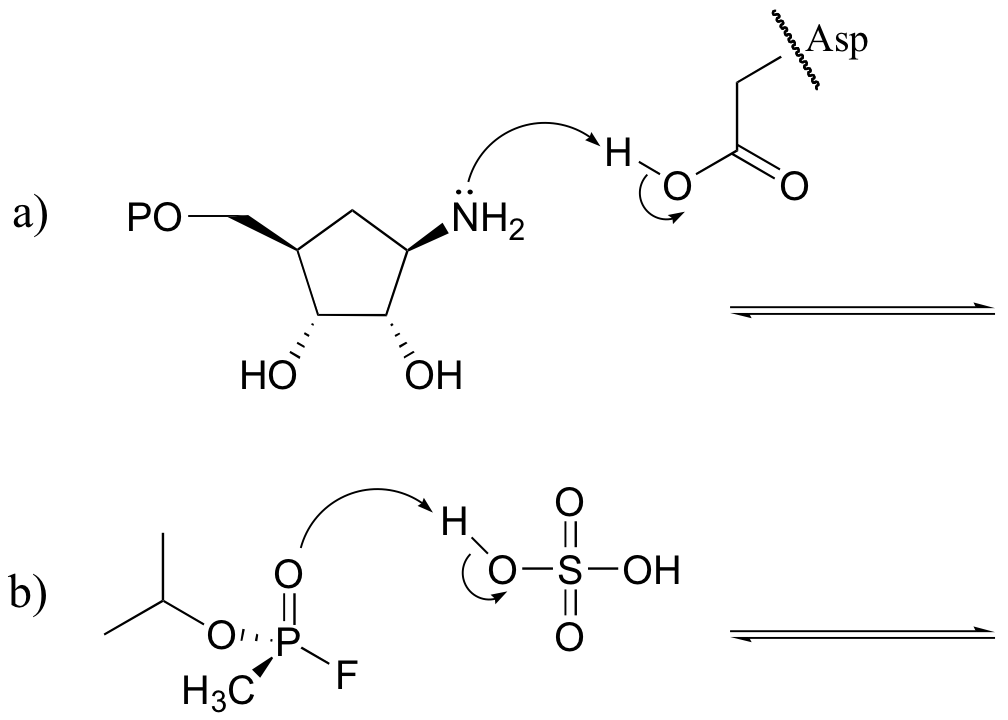
Exercise 7.1: Draw structures for the missing conjugate acids or conjugate bases in the reactions below.
7.1B: The Lewis definition of acidity
The Brønsted-Lowry picture of acids and bases as proton donors and acceptors is not the only definition in common use. A broader definition is provided by the Lewis theory of acids and bases, in which a Lewis acid is an electron-pair acceptor and a Lewis base is an electron-pair donor. This definition covers Brønsted-Lowry proton transfer reactions, but also includes reactions in which no proton transfer is involved. The interaction between a magnesium cation (Mg+2) and a carbonyl oxygen is a common example of a Lewis acid-base reaction. The carbonyl oxygen (the Lewis base) donates a pair of electrons to the magnesium cation (the Lewis acid).
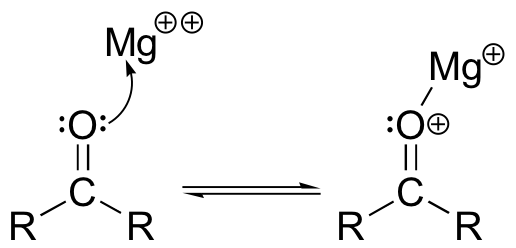
As we will see in chapter 11 when we begin the study of reactions involving carbonyl groups, this interaction has the very important effect of increasing the polarity of the carbon-oxygen double bond.
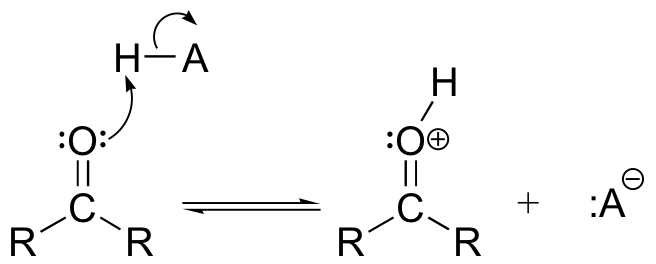
The Brønsted-Lowry equivalent of the reaction above is simply protonation of the carbonyl group. This, too, has the effect of increasing the polarity of the carbonyl double bond.
While it is important to be familiar with the Lewis definition, the focus throughout the remainder of this chapter will be on acid-base reactions of the Brønsted-Lowry type, where an actual proton transfer event takes place.


