7.4: Más sobre los efectos de resonancia en la acidez y basicidad
- Page ID
- 2363
7.4A: The acidity of phenols
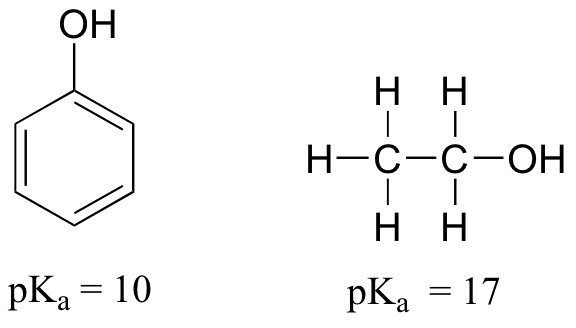
Resonance effects involving aromatic structures can have a dramatic influence on acidity and basicity. Notice, for example, the difference in acidity between phenol and ethanol.
Looking at the conjugate base of phenol, we see that the negative charge can be delocalized by resonance to three different carbons on the aromatic ring.
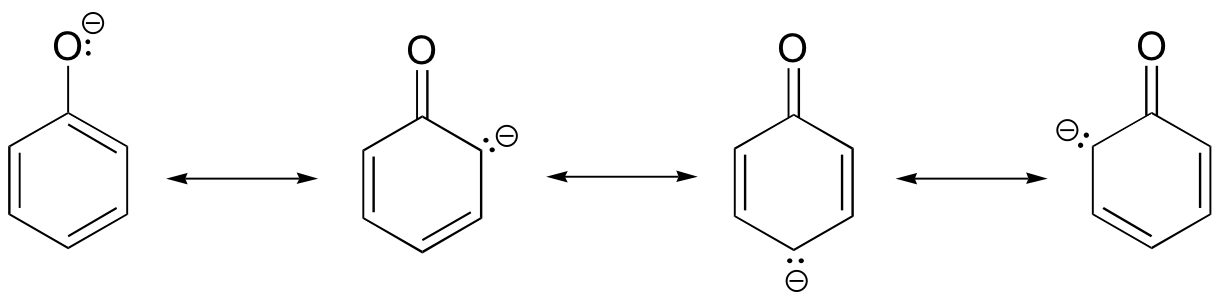
Although these are all minor resonance contributors (negative charge is placed on a carbon rather than an oxygen), they nonetheless have a significant effect on the acidity of the phenolic proton. Essentially, the benzene ring is acting as an electron-withdrawing group.
As we begin to study in detail the mechanisms of biological organic reactions, we’ll see that the phenol side chain of the amino acid tyrosine, with its relatively acidic pKa of 9-10, often acts as a catalytic proton donor/acceptor in enzyme active sites.
Delocalization of electron density has a stabilizing effect, and the greater area over which the delocalization is possible, the greater the stabilization. The most acidic of the three phenolic groups in resveretrol (an antioxidant compound in red wine) is indicated with an arrow in the figure below. This proton is expected to have a pKa value somewhat lower than that of phenol.
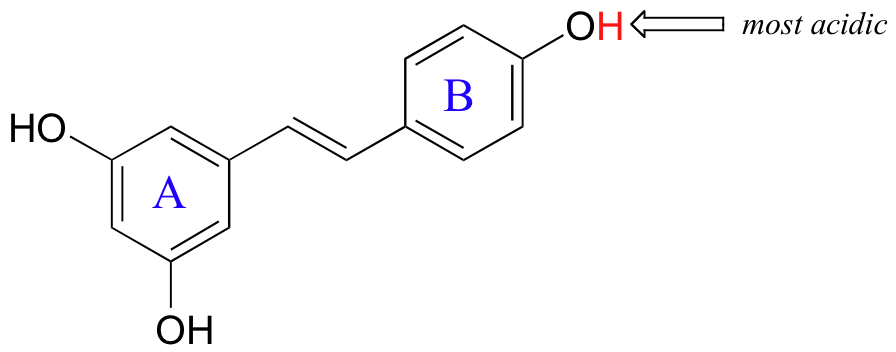
We can predict this because, when we draw all the possible resonance contributors for the conjugate base, we see that the negative charge can be delocalized over the entire π structure of the molecule, to the A ring as well as to the B ring. A negative charge that results from deprotonation of either of the other two phenol groups, however, can be delocalized only over the A ring.
| Example 7.9 |
|---|
| Indicate, on a structural drawing of the conjugate base of resveretrol, all of the atoms to which the negative charge can be delocalized by resonance. |
The base-stabilizing effect of an aromatic ring can also be accentuated by the presence of an additional electron-withdrawing substituent, such as a carbonyl. For the conjugate base of the phenol derivative below, an additional resonance contributor can be drawn in which the negative formal charge is placed on the carbonyl oxygen.

Now the negative charge on the conjugate base can be spread out over two oxygens (in addition to three aromatic carbons). The aromatic aldehyde above is expected to be substantially more acidic than phenol.
The position of the electron-withdrawing substituent relative to the phenol hydroxide is very important. When a carbonyl is in the para position (three carbons away) or the ortho position (one carbon away), it can act as an electron-withdrawing group by resonance.
In the meta position, however (two carbons away), we cannot draw a resonance contributor in which the negative formal charge is placed on the carbonyl oxygen.

In the meta position, the carbonyl still acts as an electron-withdrawing group, but only by induction, not by resonance. As stated earlier, inductive effects are generally much weaker than resonance effects.
In the laboratory, nitro groups, which are very powerfully electron-withdrawing, are often used to increase the acidity of phenol groups. Picric acid, for example, has a pKa of 0.25 – it is more acidic than trifluoroacetic acid.
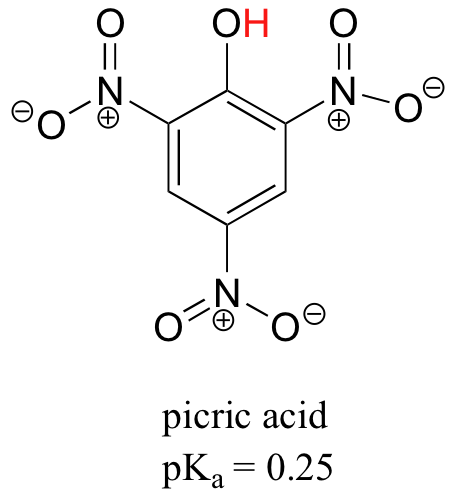
| Example 7.10 |
|---|
| Exercise 7.10: Use a resonance argument to explain why picric acid has such a low pKa. |
A methoxy group located at the para or ortho position of phenol causes a decrease in acidity. Consider the example of para-methoxyphenol.
At first inspection, you might assume that the methoxy substituent, with its electronegative oxygen, is a electron-withdrawing group by induction. That is correct, but only to a point. The oxygen atom does indeed exert an electron-withdrawing inductive effect, but the lone pairs on the oxygen cause the exact opposite effect – the methoxy group is an electron-donating group by resonance. A resonance contributor can be drawn in which a formal negative charge is placed on the carbon adjacent to the negatively-charged phenolate oxygen.
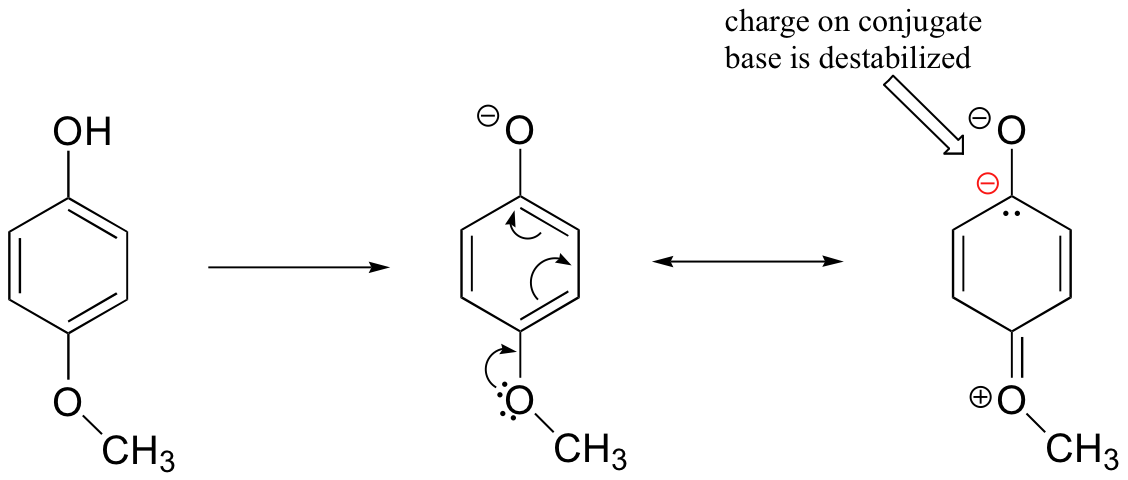
Because of like-charge repulsion, this destabilizes the negative charge on the phenolate oxygen, making it more reactive. It may help to visualize the methoxy group ‘pushing’ electrons towards the basic lone pair of the phenolate oxygen, causing them to want to reach out and grab a proton.
7.4B: The basicity of nitrogen-containing groups: aniline, imines, pyridine, and pyrrole
We have already learned that amines are relatively basic groups, while amides, due to resonance with the adjacent carbonyl, are not basic (see section 7.3B). Now, let's consider some other nitrogen-containing molecules in which aromatic groups exert an influence on basicity. When evaluating the basicity of a nitrogen-containing organic functional group, the central question we need to ask ourselves is: how reactive (and thus how basic) is the lone pair on the nitrogen? In other words, how much does that lone pair want to break away from the nitrogen nucleus and form a new bond with a hydrogen?
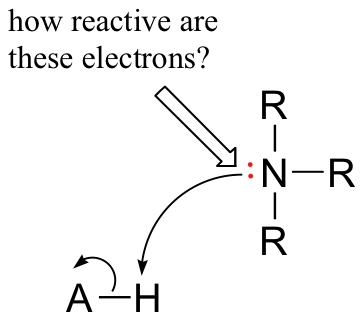
Aniline is substantially less basic than methylamine, as is evident by looking at the pKa values for their respective ammonium conjugate acids:
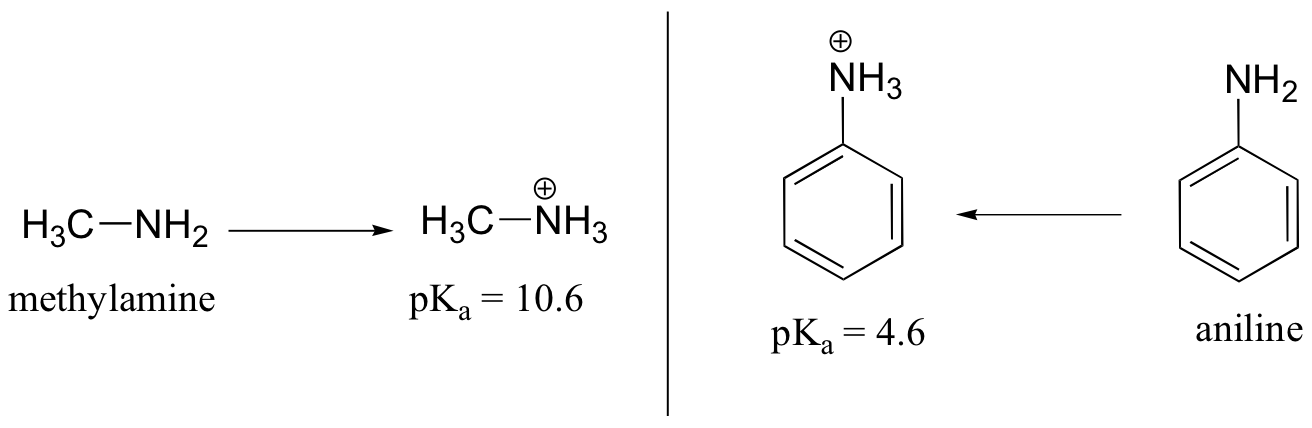
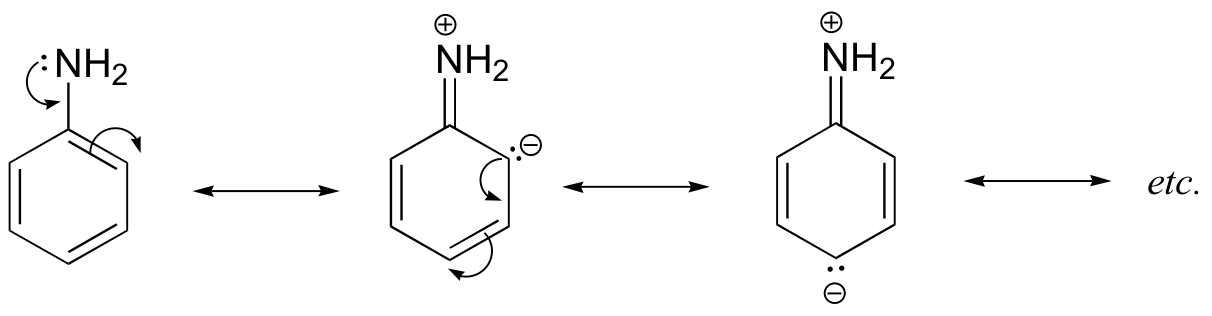
This difference is basicity can be explained by the observation that, in aniline, the basic lone pair on the nitrogen is to some extent tied up in – and stabilized by – the aromatic p system.
This effect is accentuated by the addition of an electron-withdrawing group such as a carbonyl, and reversed to some extent by the addition of an electron-donating group such as methoxide.
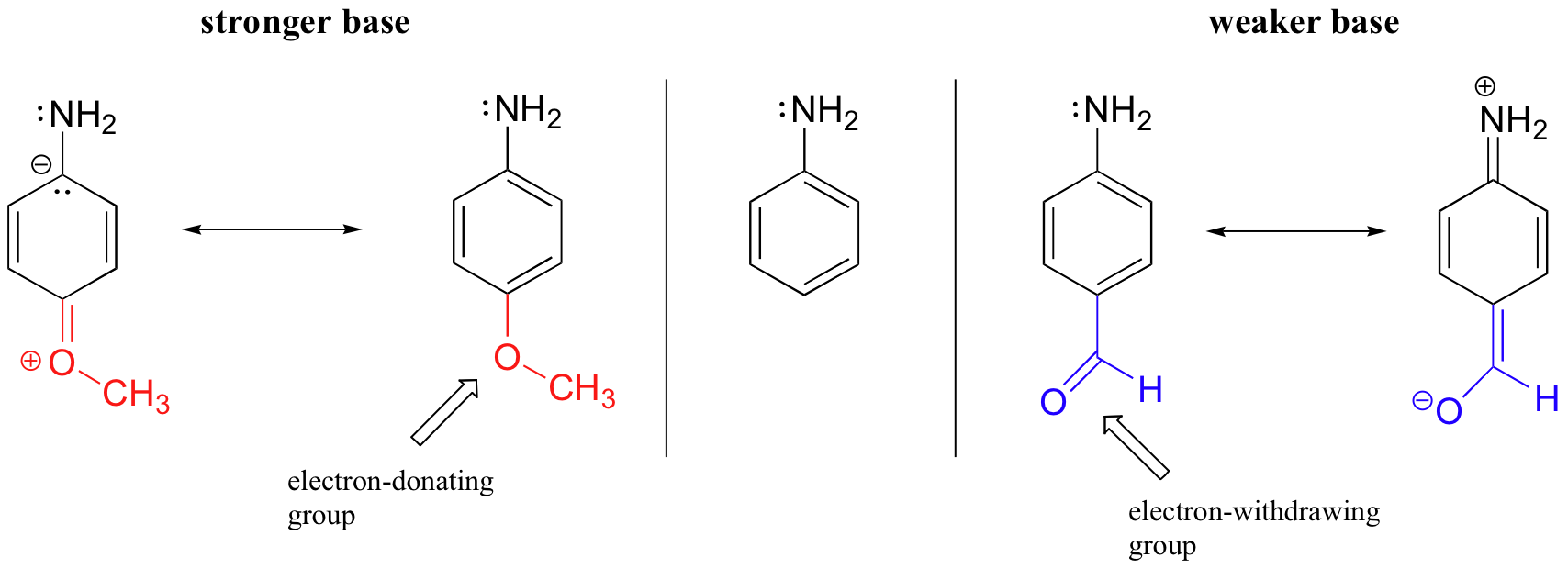
In the case of 4-methoxy aniline (the molecule on the left side of the figure above), the lone pair on the methoxy group donates electron density to the aromatic system, and a resonance contributor can be drawn in which a negative charge is placed on the carbon adjacent to the nitrogen, which makes the lone pair of the nitrogen more reactive. In effect, the methoxy group is 'pushing' electron density towards the nitrogen. Conversely, the aldehyde group on the right-side molecule is 'pulling' electron density away from the nitrogen, decreasing its basicity.
At this point, you should draw resonance structures to convince yourself that these resonance effects are possible when the substituent in question (methoxy or carbonyl) is located at the ortho or para position, but not at the meta position.
| Example 7.11 |
|---|
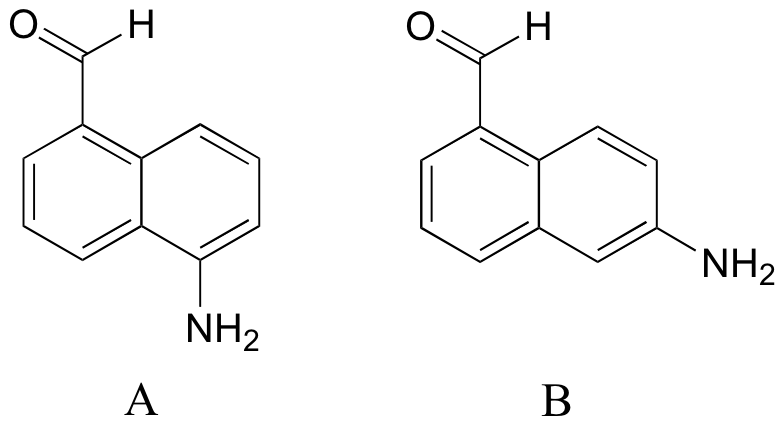
| Exercise 7.11: Which of the two aromatic aniline-like amines below is expected to be more basic? Explain your reasoning.
|
Recall from section 1.4A that an imine functional group is characterized by an sp2-hybridized nitrogen double-bonded to a carbon. Imines are somewhat basic, with pKa values for the protonated forms ranging around 7. Notice that this is significantly less basic than amine groups (eg. pKa = 10.6 for methylammonium), in which the nitrogen is sp3-hybridized. This phenomenon can be explained using orbital theory and the inductive effect: the sp2 orbitals of an imine nitrogen are one part s and two parts p, meaning that they have about 67% s character. The sp3 orbitals of an amine nitrogen, conversely, are only 25% s character (one part s, three parts p). Because the s atomic orbital holds electrons in a spherical shape, closer to the nucleus than a p orbital (section 1.1A), sp2hybridization implies greater electronegative than sp3 hybridization. Finally, recall the inductive effect from section 7.3C: more electronegative atoms absorb electron density more easily, and thus are more acidic. Moral of the story: protonated imine nitrogens are more acidic than protonated amines, thus imines are less basic than amines.
When a nitrogen atom is incorporated directly into an aromatic ring, its basicity depends on the bonding context. In a pyridine ring, for example, the nitrogen lone pair occupies an sp2-hybrid orbital, and is not part of the aromatic sextet - it is essentially an imine nitrogen. Its electron pair is available for forming a bond to a proton, and thus the pyridine nitrogen atom is somewhat basic.

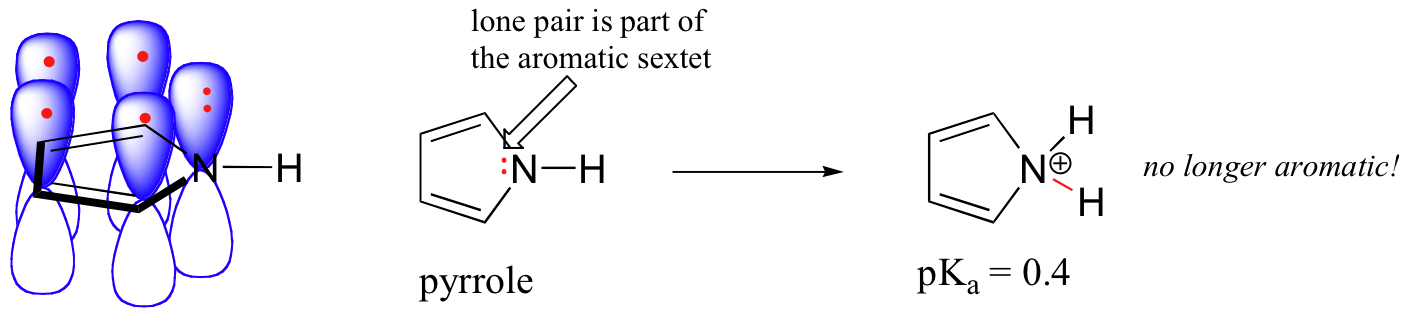
In a pyrrole ring, in contrast, the nitrogen lone pair is part of the aromatic sextet. This means that these electrons are very stable right where they are (in the aromatic system), and are much less available for bonding to a proton (and if they do pick up a proton, the aromic system is destroyed). For these reasons, pyrrole nitrogens are not strongly basic.
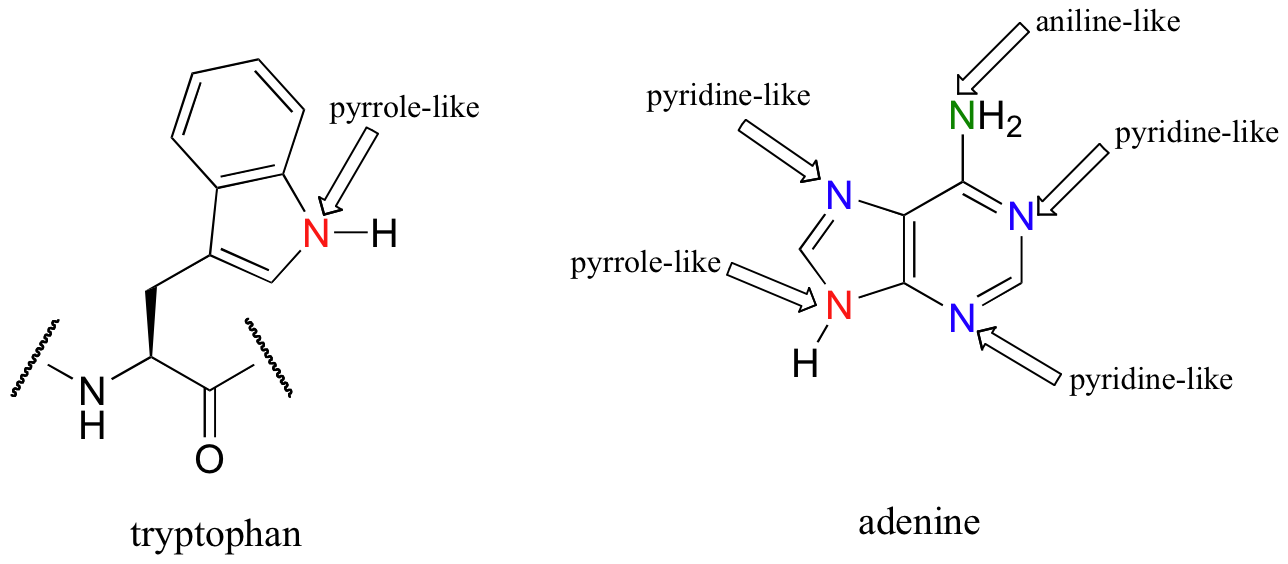
The aniline, pyridine, and pyrrole examples are good models for predicting the reactivity of nitrogen atoms in more complex ring systems (a huge diversity of which are found in nature). The tryptophan side chain, for example, contains a non-basic 'pyrrole-like' nitrogen, while adenine (a DNA/RNA base) contains all three types.