5.8: NMR de moléculas fosforiladas
- Page ID
- 2349
Because so many biological molecules contain phosphoryl groups, it is worthwhile to look at how scientists use NMR to determine the structure of these molecules. Consider the case of isopentenyl diphosphate, the building block molecule used by cells to make 'isoprenoid' compounds such as cholesterol (in many animals), or beta-carotene (in some plants). NMR spectra of this molecule were taken in a D2O solvent, buffered with ND4OD (the deuterium equivalent of aqueous ammonium hydroxide, NH4OH) (J. Org. Chem. 1986, 51, 4768).
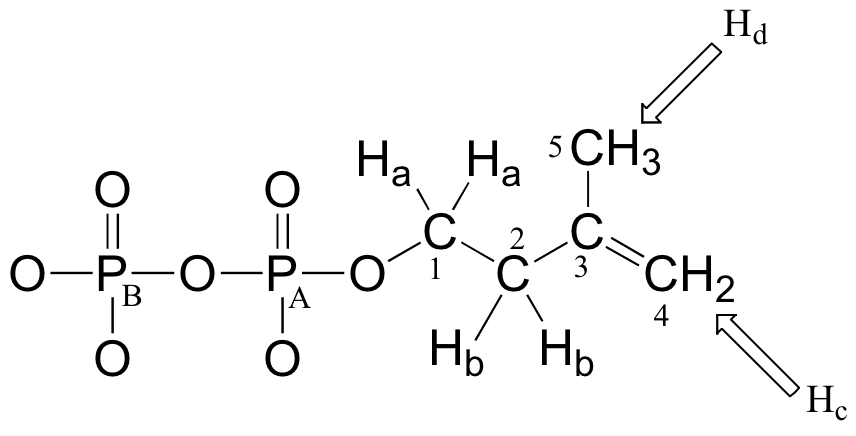
1H-NMR
Ha: 4.05 ppm (td); 3JHa-Hb = 6.6 Hz; 3JHa-PA= 3.3 Hz.
Hb: 2.39 ppm (t) 3JHa-Hb = 6.6 Hz
Hc: 4.86 ppm (s)
Hd: 1.77 ppm (s)
The 1H-NMR signals for Hb, Hc, and Hd are what we would expect given our present knowledge. Why, though, is the signal for Ha split into a triplet of doublets (td)? First of all, as, expected, the two neighboring Hb protons split the Ha signal into a triplet, with 3J = 6.6 Hz. Then, the signal is further split into doublets (3J = 3.3 Hz) by PA, the closer of the two phosphorus atoms.
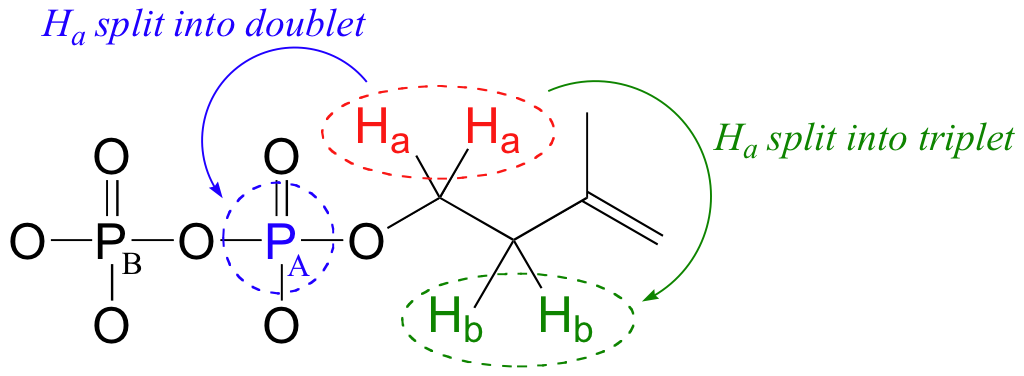
Recall that the most abundant isotope of phosphorus is 31P, which is NMR active - it has a magnetic dipole just like 13C and 1H, and thus will participate in spin-spin coupling interactions with nearby protons and 13C nuclei.
In the 13C spectrum, notice that the signals for both C1 and C2 are split into doublets by the magnetic field of PA, through 2-bond and 3-bond coupling, respectively.
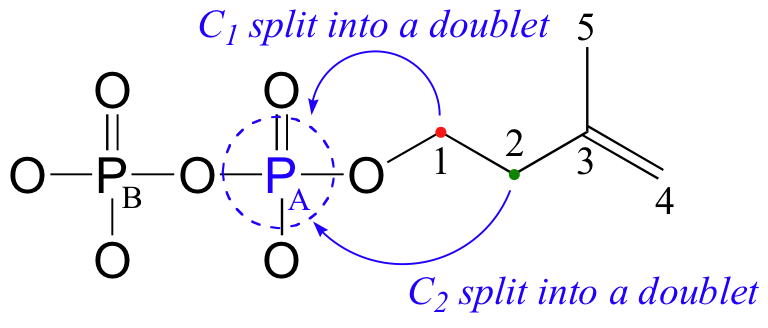
13C-NMR (proton-decoupled)
C1: 40.7 ppm (d); 2JC1-PA= 7.2 Hz
C2: 67.0 ppm (d); 3JC2-PA= 4.0 Hz
C3: 147.4 ppm
C4: 114.6 ppm
C5: 24.5 ppm
This splitting is observed even in the proton-decoupled spectrum - carbon-phosphorus coupling is not turned off.
Because 31P is NMR-active, we can also, with the right instrumentation, directly observe the phosphorus NMR signals. In an NMR instrument with a 7.1 Tesla magnet, where protons resonate at 300 MHz and 13C nuclei resonate at 75 MHz, phosphorus resonates at 32 MHz. In 31P-NMR experiments, the reference standard used to determine the 0 ppm point is usually phosphoric acid (TMS, the standard for 1H- and 13C-NMR, doesn't have a phosphorus atom!). The 31P-NMR spectrum of isopentenyl diphosphate has, as expected, two peaks, each of which is upfield of the phosphoric acid standard (negative chemical shifts!) and split into a doublet (2JP-P = 20 Hz) due to coupling between the two phosphorus nuclei.
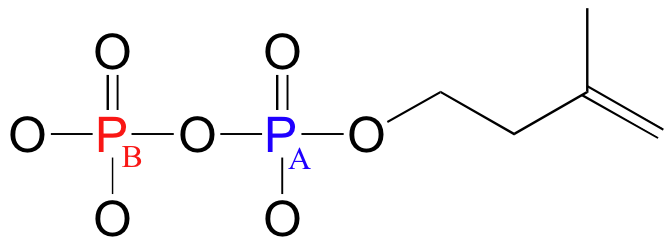
PA: -11.03 ppm (d)
PB: -7.23 ppm (d)
2J PA-PB= 20 Hz
Notice that although the C1 and C2 signals were split by PA in our 13C-NMR spectrum, in the 31P-NMR spectrum the PA signal is not split by the nearby carbons - this is because, of course, both of these carbons are the NMR-inactive 12C isotope in 99 out of 100 molecules. In addition, P-H splitting is not observed in this 31P spectrum, because proton decoupling is in effect.


