11.1: Prefix
- Page ID
- 2390
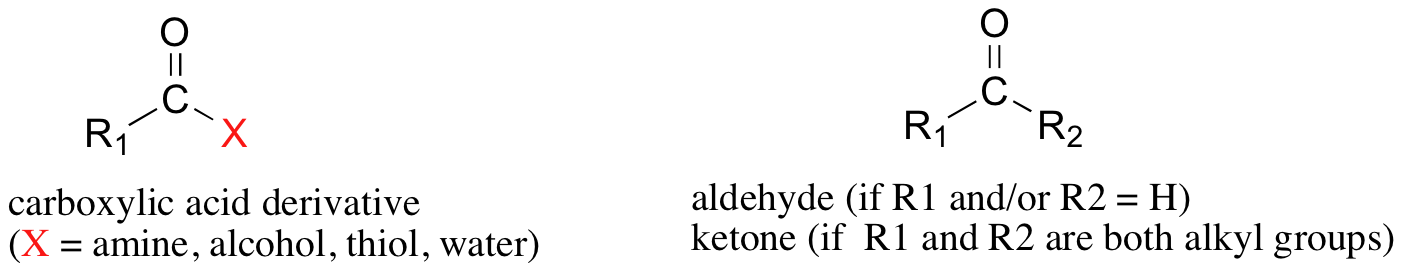
If you look through any biochemistry textbook, you will see example after example of organic compounds that contain carbonyl groups, where a carbon atom is double bonded to an oxygen atom. Carbonyl functional groups fall into two basic categories: carboxylic acid derivatives and ketones/aldehydes. In carboxylic acid derivatives - a group which includes amides, esters, thioesters, and acyl phosphates in addition to carboxylic acids - the carbonyl carbon is bonded on one side to a carbon and on the other side to a heteroatom (nitrogen, oxygen, or sulfur).
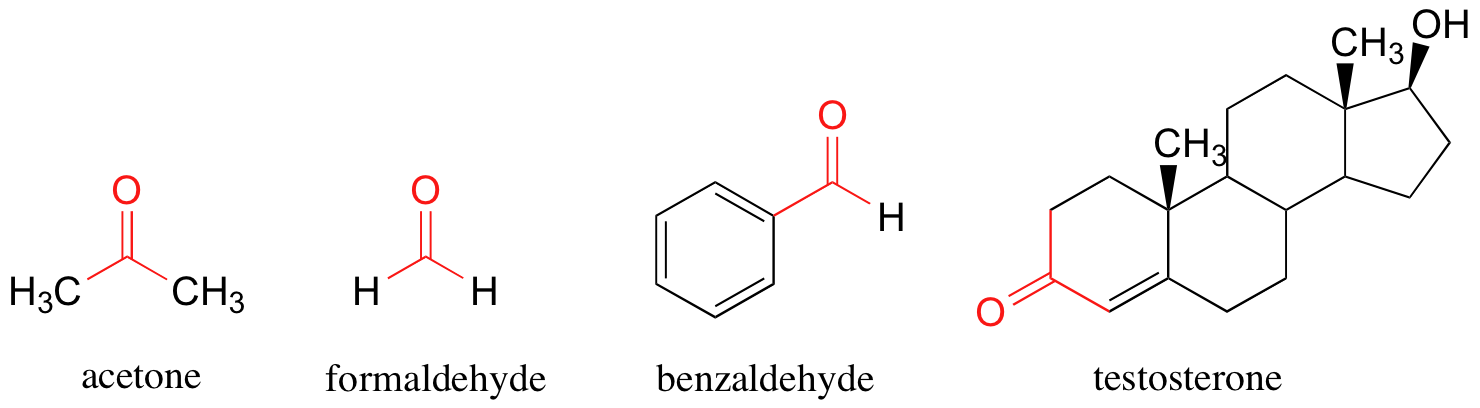
When the neighboring atoms are both carbons or hydrogens, the group is referred to as a ketone (if both atoms are carbons) or an aldehyde (if one or both atoms is a hydrogen). A few examples of ketones and aldehydes are shown below. You probably are familiar with them: acetone, the simplest ketone compound, is the solvent in nail polish remover, benzaldehyde is the flavoring in maraschino cherries, and formaldehyde (a special case in which the carbonyl carbon is bonded to hydrogens on both sides) is the nasty-smelling stuff that was used to preserve the unlucky frog that you dissected in high school biology class. Testosterone, which contains alcohol and alkene functional groups as well as a ketone group, is a male sex hormone.
In carboxylic acid derivatives, the heteroatom bonded to the carbonyl carbon on one side is a potential leaving group, whereas in aldehydes and ketones the hydrogen and/or carbon is definitely not a good leaving group, so the two categories of compounds react in fundamentally different ways. In this chapter we will learn about how aldehydes and ketones react, then in the following chapter we will focus on the chemistry of carboxylic acid derivatives.


