12.5: Ésteres
- Page ID
- 2404
- 12.4A: Nonenzymatic esterification: synthesis of ‘banana oil’
- 12.4B: Nonenzymatic ester hydrolysis and the soap-making process
- 12.4C: Enzymatic ester hydrolysis: acetylcholinesterase and sarin nerve gas
- 12.4D: More enzymatic ester hydrolysis: lipase, the resolution of enantiomers, and dehalogenation
- 12.4E: Transesterification: the chemistry of aspirin and biodeisel
12.4A: Nonenzymatic esterification: synthesis of ‘banana oil’
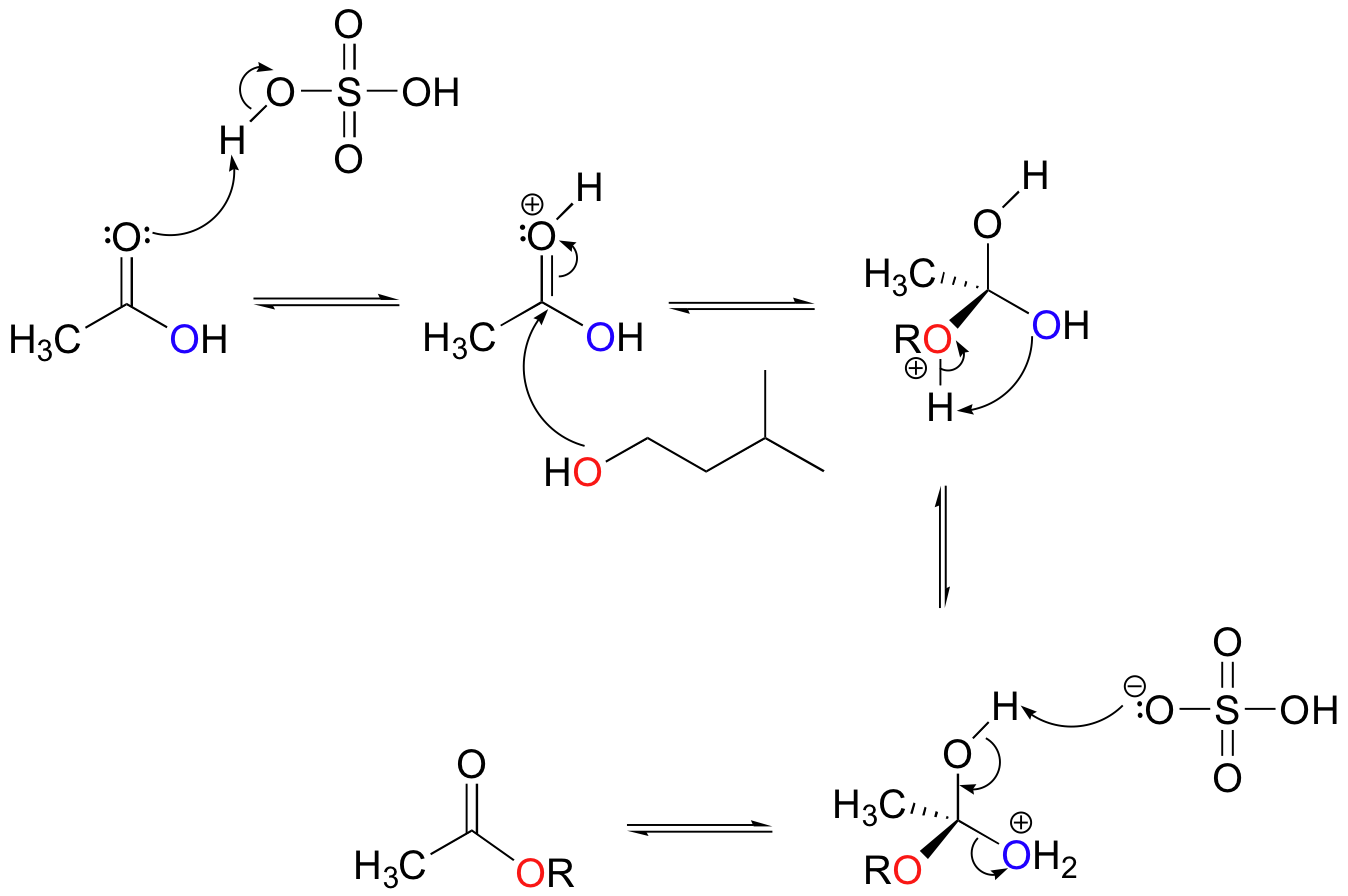
We have already seen an example of enzyme-catalyzed ester synthesis in the monoacylglycerolacyltransferase reaction (section 12.3C). In that case, the carboxylate group of a fatty acid had to be activated before the esterification reaction could take place. In the laboratory, of course, synthetic chemists are free to adjust the pH of a reaction, adding acid or base as appropriate. Although carboxylic acids need to be converted to acid chlorides or another reactive derivative in order to convert them to amides (see section 12.2D), many esters can be synthesized simply by mixing together a carboxylic acid and an alcohol, along with a catalytic amount of sulfuric acid. A popular reaction in undergraduate organic lab courses is the preparation of isopentyl acetate (also called ‘banana oil’ because it is a flavor component in bananas) from acetic acid and isopentyl alcohol.
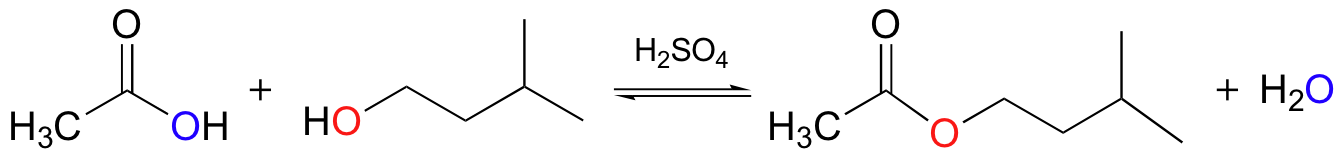
Notice the double arrows in the above figure: because esters and carboxylic acids are of approximately the same stability, this esterification reaction is highly reversible.
The main role of the acid catalyst in this reaction is to protonate the carboxylic acid, thus making the carbonyl carbon more electrophilic.
Exercise 12.4: In the synthesis of isopentyl acetate, an excess of acetic acid is used, usually about three molar equivalents relative to the amount of isopentyl alcohol. What is the reason for this?
Exercise 12.5: Could a similar esterification reaction occur with the addition of a small amount of NaOH instead of H2SO4? Explain.
Exercise 12.6: Draw a complete mechanism for the exact reverse of the acid-catalyzed esterification shown above. What would you call this reaction in organic chemistry terms?
Many other fragrant esters can be synthesized in similar reactions. A few examples of esters found in foods are given below.
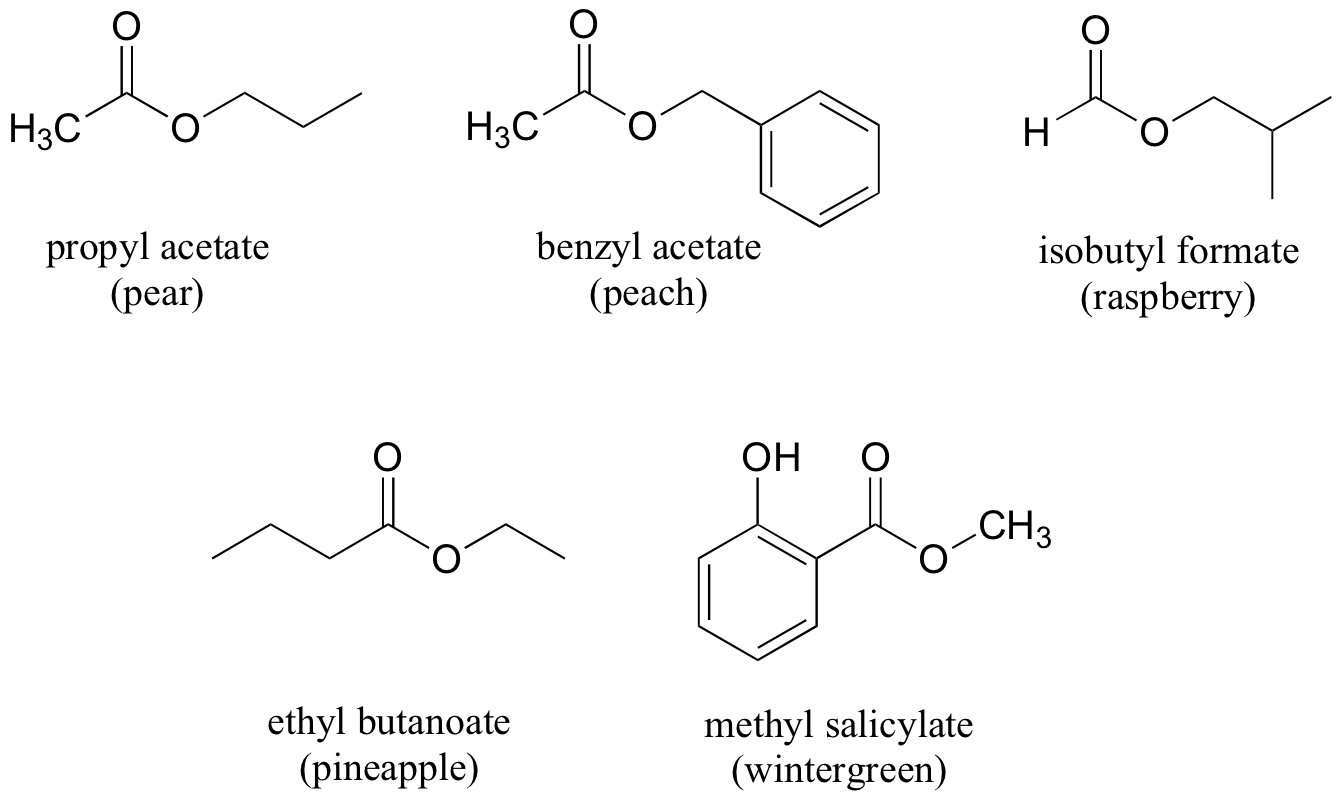
Exercise 12.7: For each fragrant ester shown above, provide the carboxylic acid and alcohol starting materials needed.
12.4B: Nonenzymatic ester hydrolysis and the soap-making process
In aqueous solution, esters are subject to hydrolysis to the corresponding carboxylic acid and alcohol components.
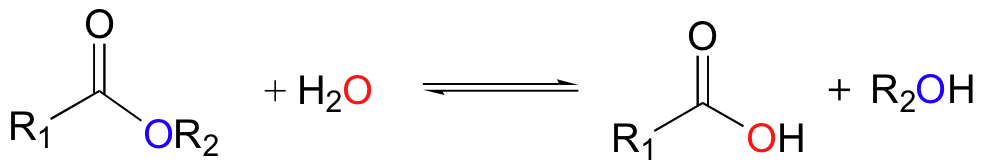
Hydrolysis is, of course, the reverse of the esterification reaction discussed above. With no enzyme or other catalyst involved, hydrolysis generally occurs slowly at neutral pH. The reaction is much faster, however, if a catalytic amount of acid or base is added. For example, the banana oil (isopentyl acetate) discussed previously will hydrolyze back to acetic acid and isopentyl alcohol if it is stirred in acidic or basic water. The addition of base accelerates ester hydrolysis because the nucleophile is a hydroxide ion, which is much more reactive than a water molecule.
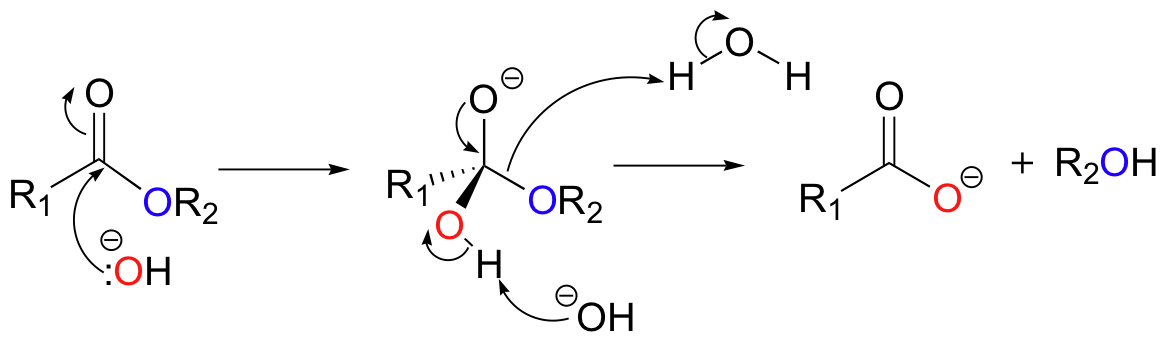
Base-catalyzed hydrolysis of esters is ancient synthetic organic chemistry, carried out by human beings for centuries in the form of soap-making. Recall from our previous discussion (section 2.3A) that soap is a mixture of glycerol and fatty acids derived from animal fats and, in more modern times, vegetable oils. The fatty acid/glycerol mixture that makes up soap is simply the product of the basic hydrolysis of triacylglycerol.
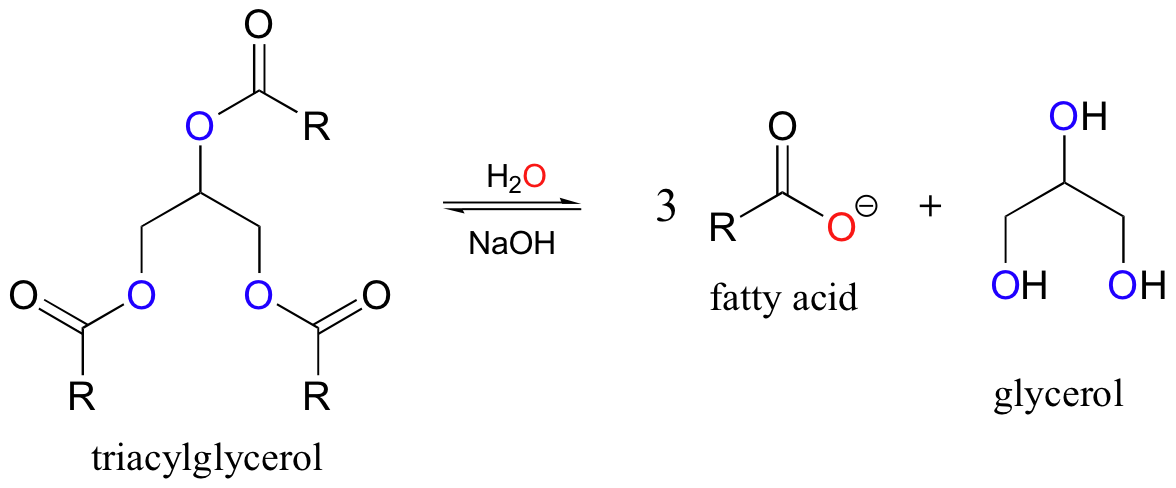
In ancient times the base came from wood ash, while in modern processes lye (sodium hydroxide) is used. Soap-making is a fun organic reaction that can be done (with the proper precautions) safely and effectively in the undergraduate laboratory.
12.4C: Enzymatic ester hydrolysis: acetylcholinesterase and sarin nerve gas
Ester hydrolysis is also an important biochemical transformation. Acetylcholinesterase, an enzyme present in the synapses between neurons, catalyzes the hydrolysis of acetylcholine, a neurotransmitter that triggers muscle contraction.
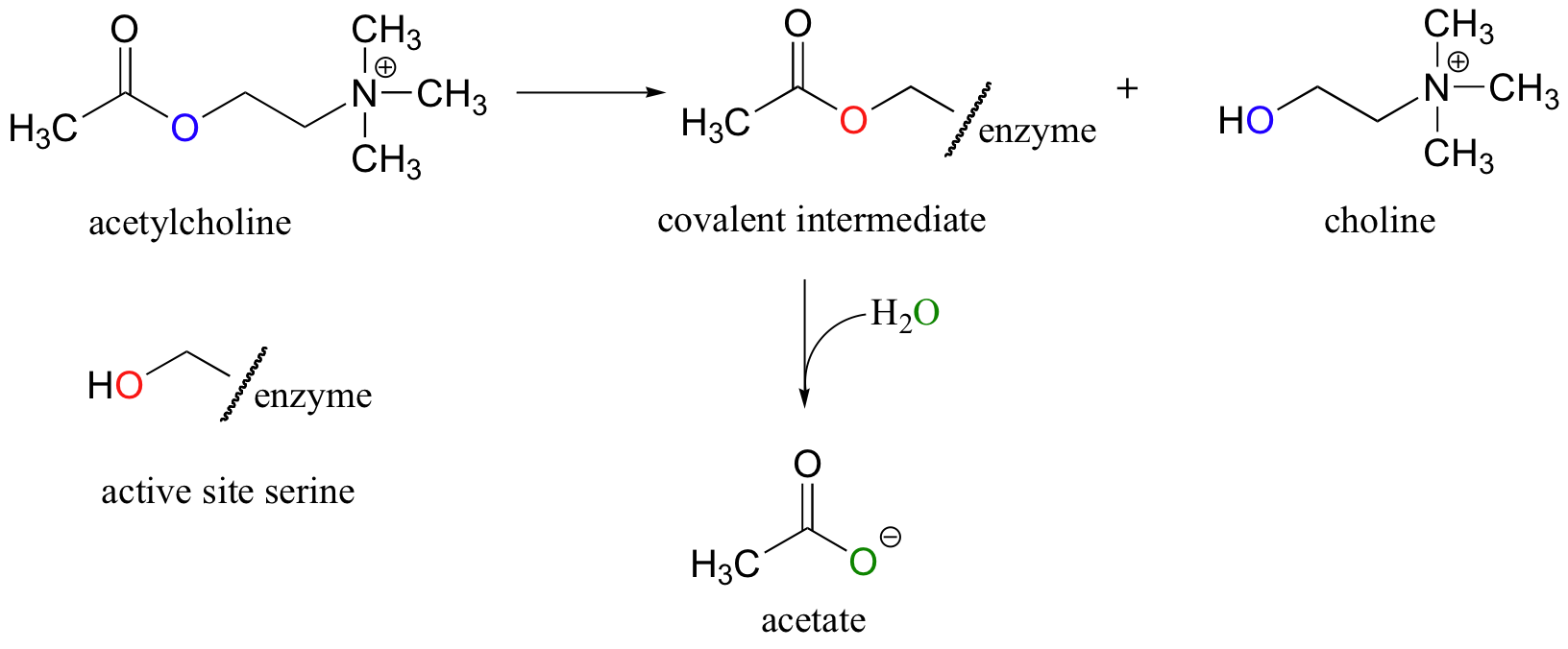
Like many hydrolytic enzymes, the reaction proceeds via a covalent enzyme-substrate intermediate, formed when the acyl group of acetylcholine is initially transferred to an active-site serine (this is a transesterification reaction). A water nucleophile then attacks this ester, driving off acetate and completing the hydrolysis. If the action of acetylcholinesterase is inhibited, acetylcholine in the synapse does not get hydrolyzed and thus continues to trigger muscle contractions, resulting in paralysis and death in severe cases. Sarin nerve gas is a potent, irreversible inhibitor (section 6.5D) of acetylcholinasterase. Some victims of the Tokyo subway sarin attack in 1995 who were exposed to low levels of the gas reported that they initially thought an eclipse of the sun had occurred, because everything looked dark when they came out of the station. This was due to uncontrolled contraction of their pupils (Murakami, H., Underground New York, Vintage International, 2001).
Sarin, is an organophosphorus compound. It inhibits acetylcholinesterase by phosphorylating the active site serine of the enzyme.
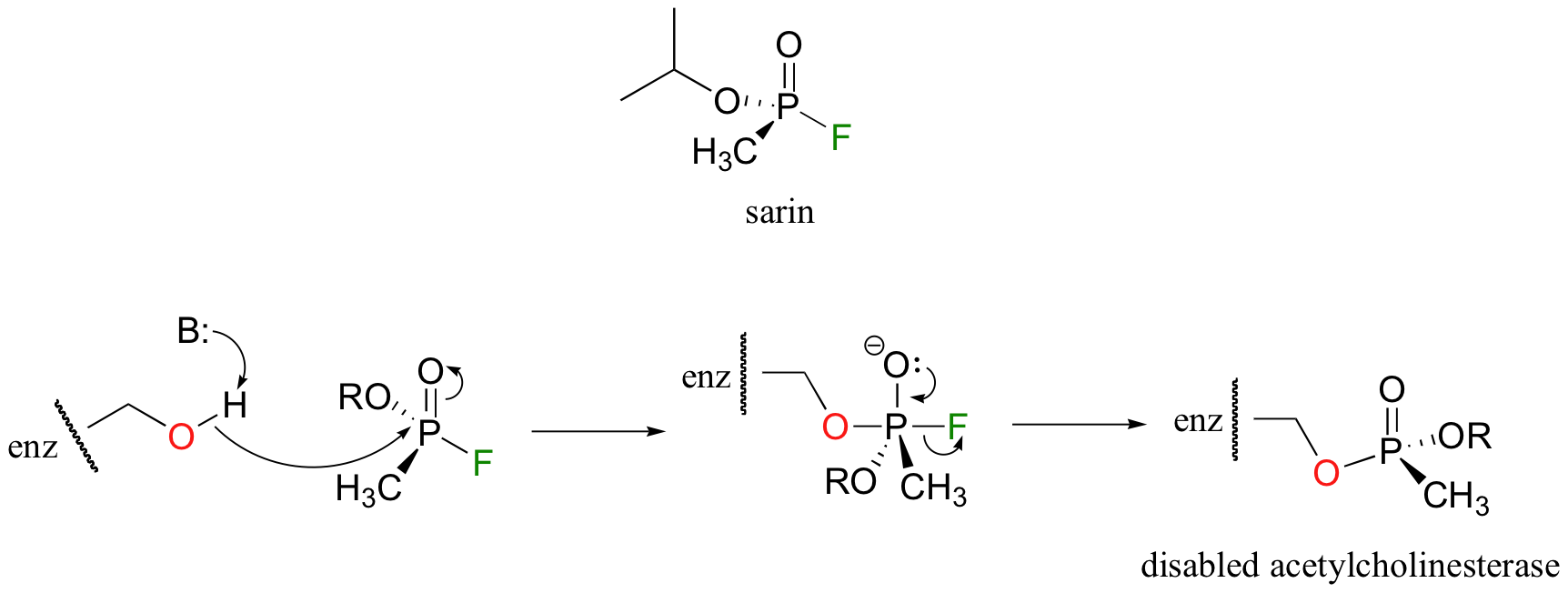
This leaves the active site serine unable to play its role in the hydrolysis of acetylcholine. In the Tokyo subway sarin attack, many lives were saved once medical personnel realized the identity of the poison gas and were able to administer atropine, an antidote that blocks the binding of acetylcholine to its receptors.
12.4D: More enzymatic ester hydrolysis: lipase, the resolution of enantiomers, and dehalogenation
Lipase is another important enzyme that catalyzes ester hydrolysis reactions. When you eat the triacylglycerols in animal fat, the first step in digestion is the release of fatty acids through hydrolysis. This is essentially the same reaction as described above in the section on soap-making, however it is carried out at neutral pH by an enzyme in the pancreas.
Aside from their utility in digesting greasy hamburgers, lipases can be valuable tools in the organic synthesis laboratory. Unlike many other enzymes, lipases have evolved to be very permissive in their recognition of substrate esters – in other words, a single enzyme will hydrolize many different esters, including non-natural (synthetic) esters made by human chemists. Lipases are, however, very stereospecific, meaning that they will often hydrolyze one enantiomer of an ester-containing compound but not the other. Using this property of lipases, chemists have developed methods to separate two enantiomeric forms of an ester through stereospecific hydrolysis (recall from section 3.2A that enantiomers cannot be separated by physical processes such as silica column chromatography, only by chiral reagents. Lipases are, of course, chiral reagents). An example of lipase-assisted separation of enantiomers is shown below. (The example is from Silverman, R. B. The Organic Chemistry of Enzyme-Catalyzed Reactions p. 62, San Diego, Academic Press 2000; J. Am. Chem. Soc. 1994, 116, 3180)
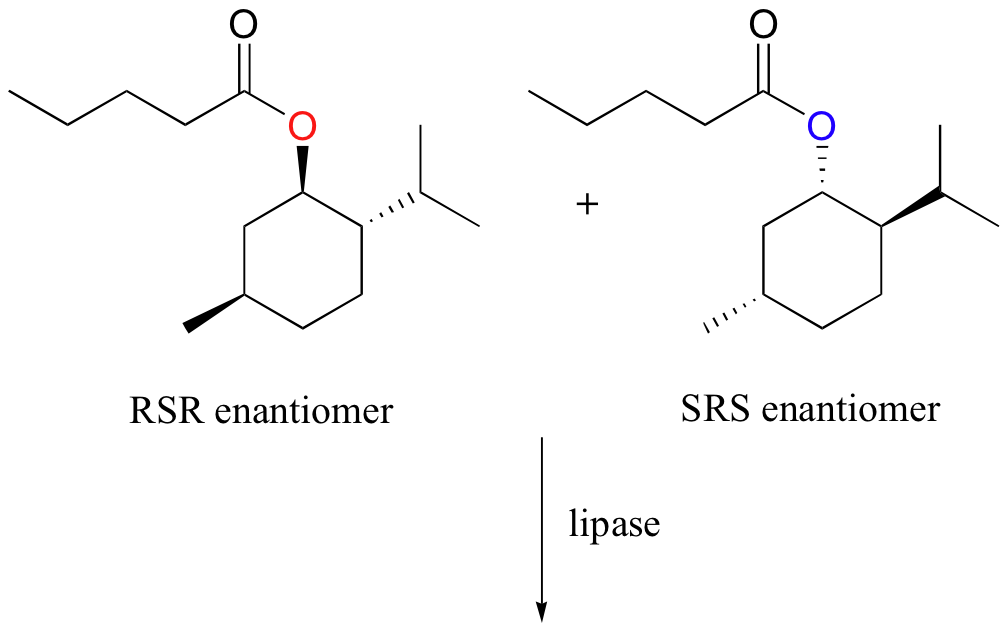
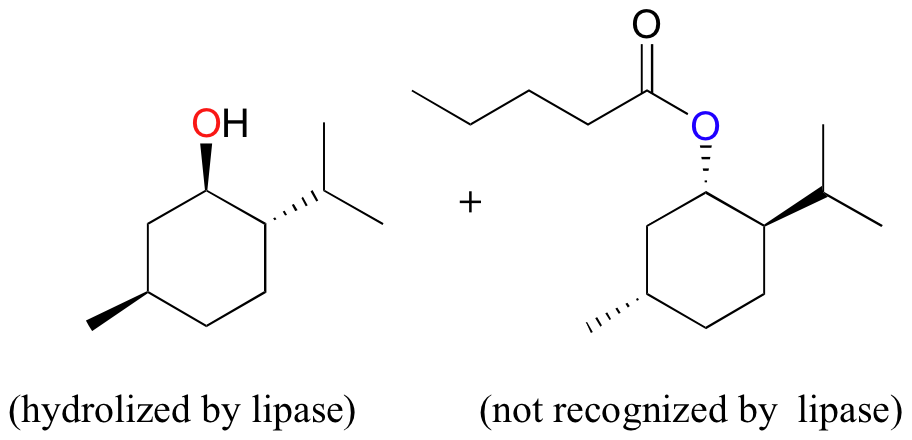
The lipase hydrolyzes only the RSR enantiomer, allowing the resulting RSR alcohol to be separated from the remaining SRS ester.
An ester hydrolysis step, in conjunction with an SN2 nucleophilic displacement, is thought to be at the heart of the mechanism of a class of bacterial enzymes called haloalkane dehalogenases. These enzymes are of interest as potential bioremediation agents, because they are capable of converting toxic haloalkanes, produced in industrial processes, into alcohols and halide ions (Biochemistry 1999, 38, 5772). The dehalogenation process begins with nucleophilic attack by an active site aspartate, which displaces a halide ion (chloride in this example).
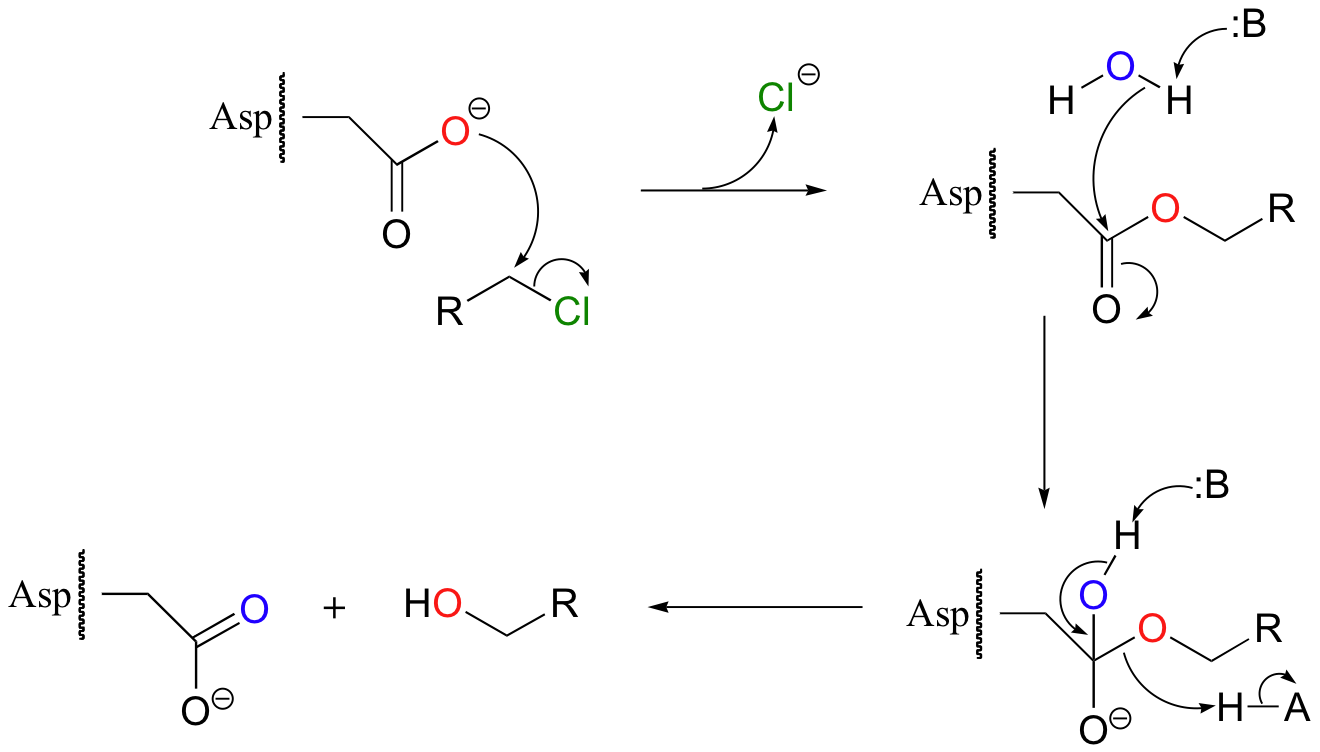
The reaction cycle is completed when the enzyme-substrate ester intermediate is hydrolized.
12.4E: Transesterification: the chemistry of aspirin and biodeisel
In a transesterification reaction, an acyl group is transferred from one alcohol to another (another way of looking at it is that one ester is being converted into another ester).
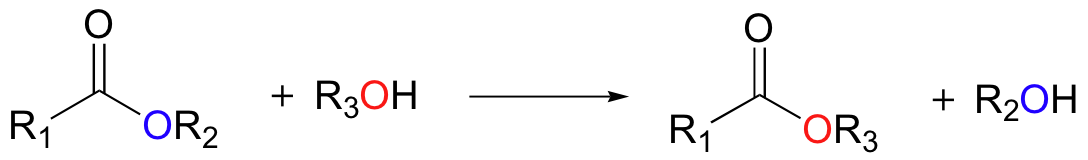
We have already seen a transesterification reaction in the first step of the acetylcholinesterase reaction (part C of this section).
If studying organic chemistry sometimes gives you a headache, you might want to turn to another transesterification reaction for help. Prostaglandins are a family of molecules that promote a wide range of biological processes, including inflammation. Acetylsalicylic acid, commonly known as aspirin, acts by transferring - through a transesterification reaction - an acetyl group to a serine residue on the enzyme responsible for the biosynthesis of prostaglandin H2 (one member of the prostaglandin family).
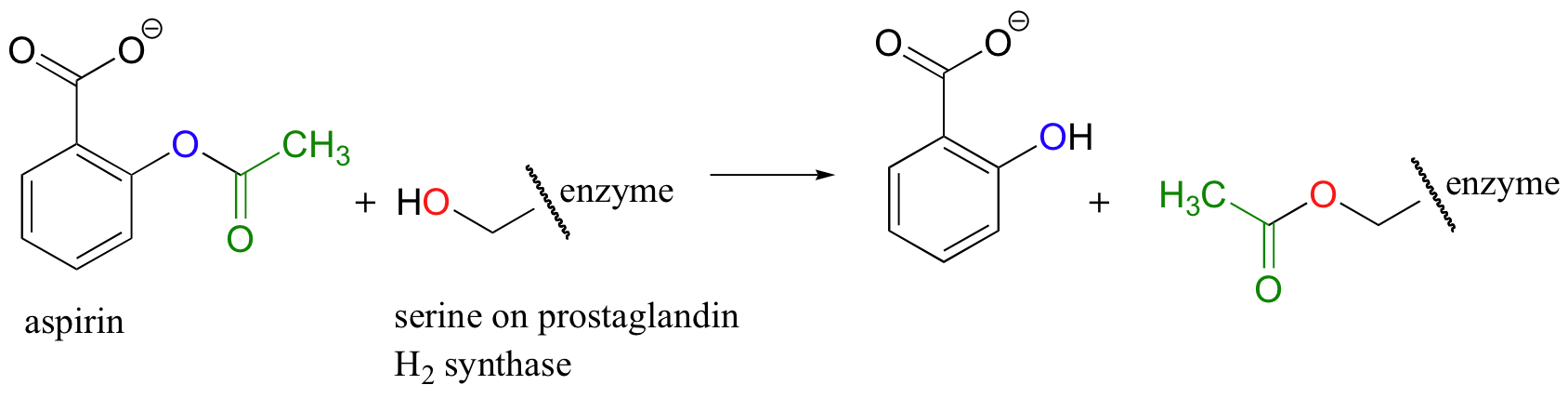
Acetylation of the serine blocks a channel leading to the active site, effectively shutting down the enzyme, impeding prostaglandin production, and inhibiting the inflammation process that causes headaches.
Exercise 12.8: Discuss the key structural features of aspirin that make it so effective at transferring its acetyl group.
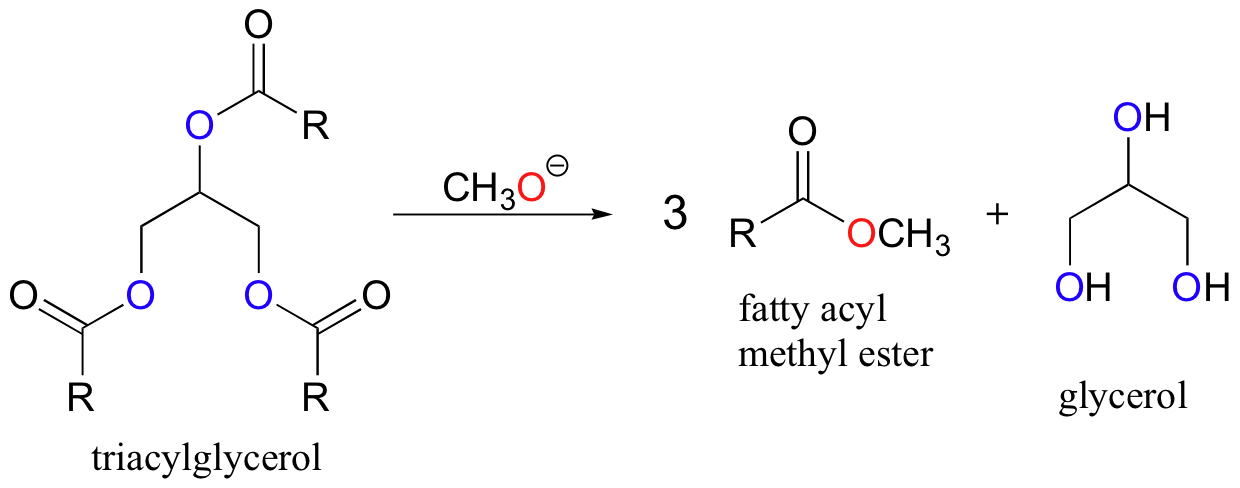
Transesterification reactions also play a key role in a technology that is already an important component in the overall effort to develop environmentally friendly, renewable energy sources. You may have heard stories about people running their cars on used french fry oil. These stories are not just urban legends! This is merely one example of the many possible applications of biofuel technology. In this process, triacylglycerols in fats and oils are transesterified with methanol (or sometimes ethanol) and a catalytic amount of sodium hydroxide. The resulting fatty acyl methyl and ethyl esters are viable motor fuels.