13.5: Reacciones Claisen
- Page ID
- 2414
The aldol reactions discussed above are nucleophilic carbonyl addition (chapter 11) reactions, in which the electrophile is the carbonyl carbon of an aldehyde or ketone. A nucleophilic enolate can also attack the carbonyl carbon of a carboxylic acid derivative in a nucleophilic acyl substitution (chapter 12) reaction.
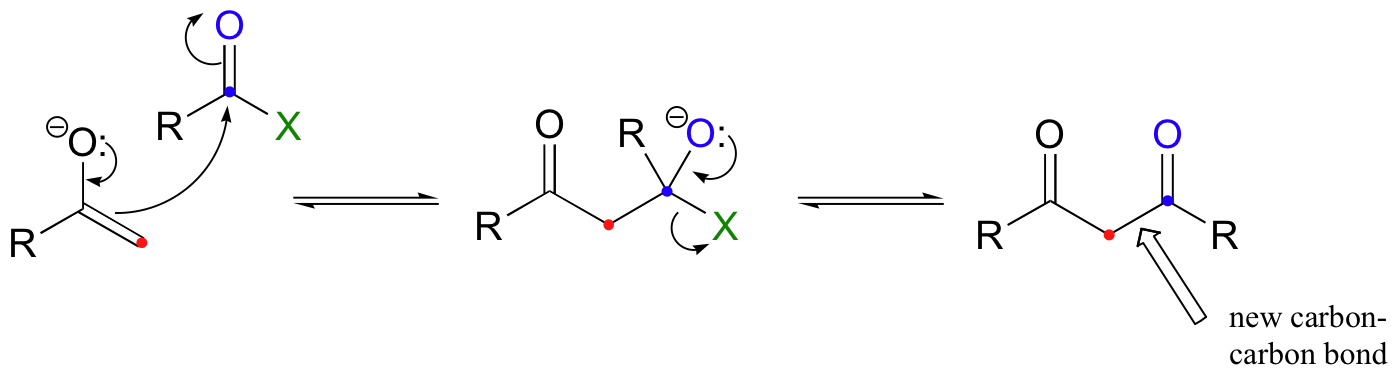
This is referred to as a Claisen condensation, after the German chemist Ludwig Claisen (1851-1930). The reverse of a Claisen condensation is called a retro-Claisen cleavage.
13.4A: Claisen condensations
An early step in the biosynthesis of cholesterol and other ‘isoprenoid’ compounds is a Claisen condensation between two acetyl CoA molecules. An initial trans-thioesterase step (section 12.3B) transfers the acetyl group of the first acetyl CoA to an enzymatic cysteine (step 1). In the Claisen condensation phase of the reaction, the alpha-carbon of a second acetyl CoA is deprotonated, forming an enolate (step 2).
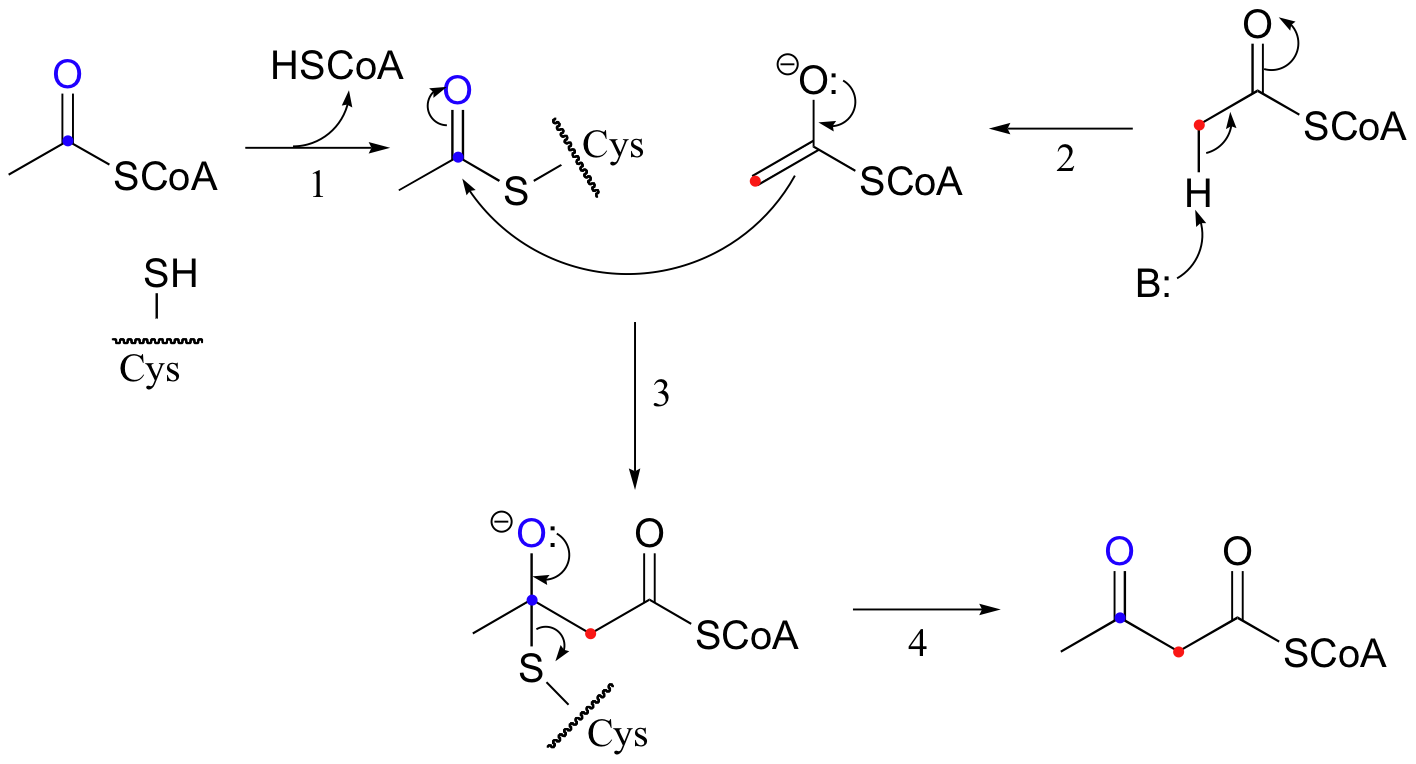
The enolate carbon attacks the electrophilic thioester carbon, forming a tetrahedral intermediate (step 3) which quickly collapses to expel the cysteine thiol (step 4).
13.4B: Retro-Claisen cleavage
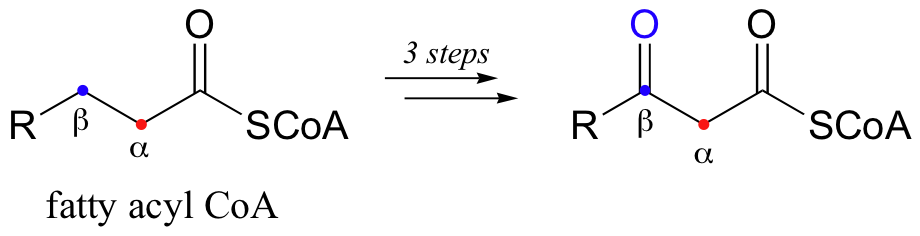
Just as there are retro-aldol cleavage reactions, there are also many examples of retro-Claisen cleavage reactions in biochemical pathways. When your body burns fat for energy, it is a retro-Claisen reaction that is responsible for breaking apart long fatty acid chains, two carbons at a time. First, a series of three reactions is required to introduce the necessary ketone group to the beta position (two of these reactions are oxidative, and will be discussed in chapter 16; the third is something that we will see in chapter 14).
What follows is the exact reverse of a Claisen condensation, with a cysteine thioeser acting as nucleophile and an enolate as the leaving group.
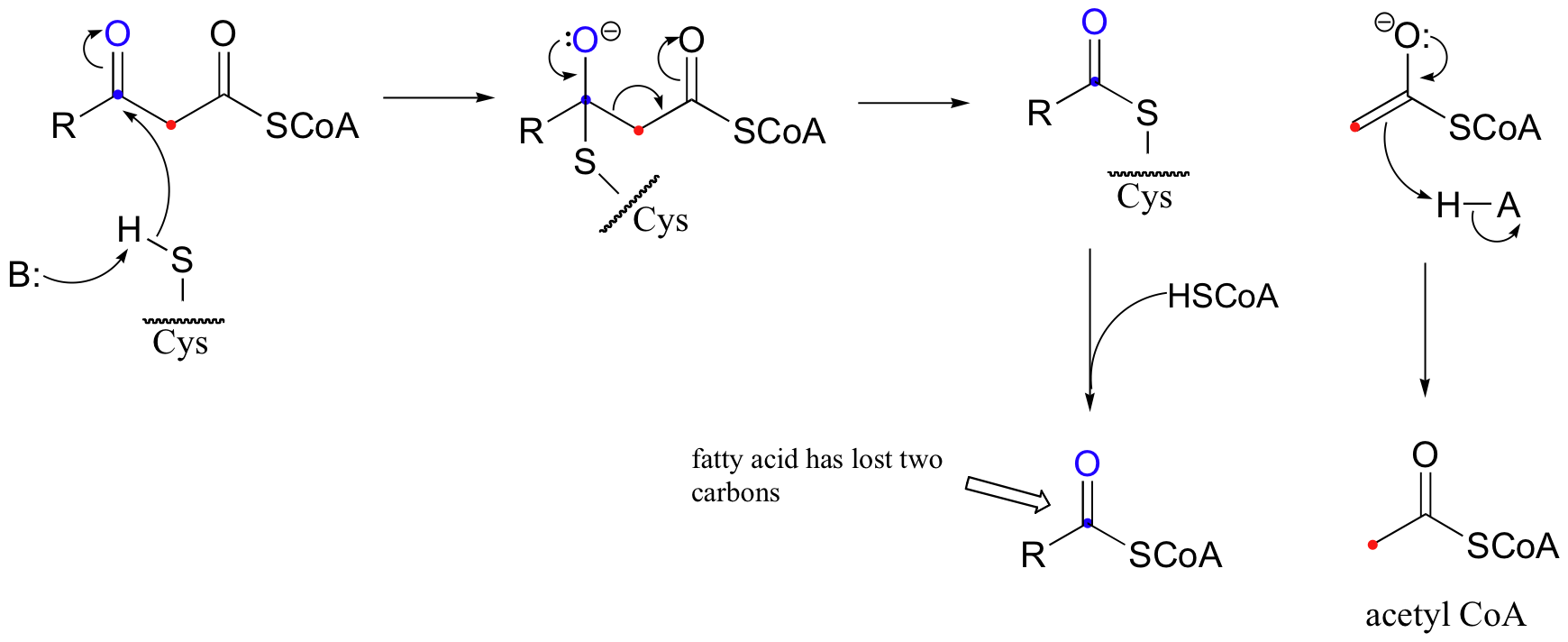
The fatty acid chain is now shorter by two carbons, and one acetyl CoA molecule is available for incorporation into the citric acid cycle, where it is further oxidized. This series of four reactions repeats itself until the entire fatty acid chain has been broken into two-carbon acetyl CoA pieces.
The reverse metabolic process - the synthesis of fatty acids from acetyl CoA - does not involve the exact reverse of this reaction. In section 13.5C we will see the slightly modified Claisen condensation reaction that is operative in the synthetic direction. You will learn when you take Biochemistry that, in most cases, degradative metabolic pathways are not the exact step-by-step reverse of the corresponding biosynthetic pathway.
A good example of a hydrolytic retro-Claisen cleavage occurs in the degradation pathway for tyrosine and phenylalanine:
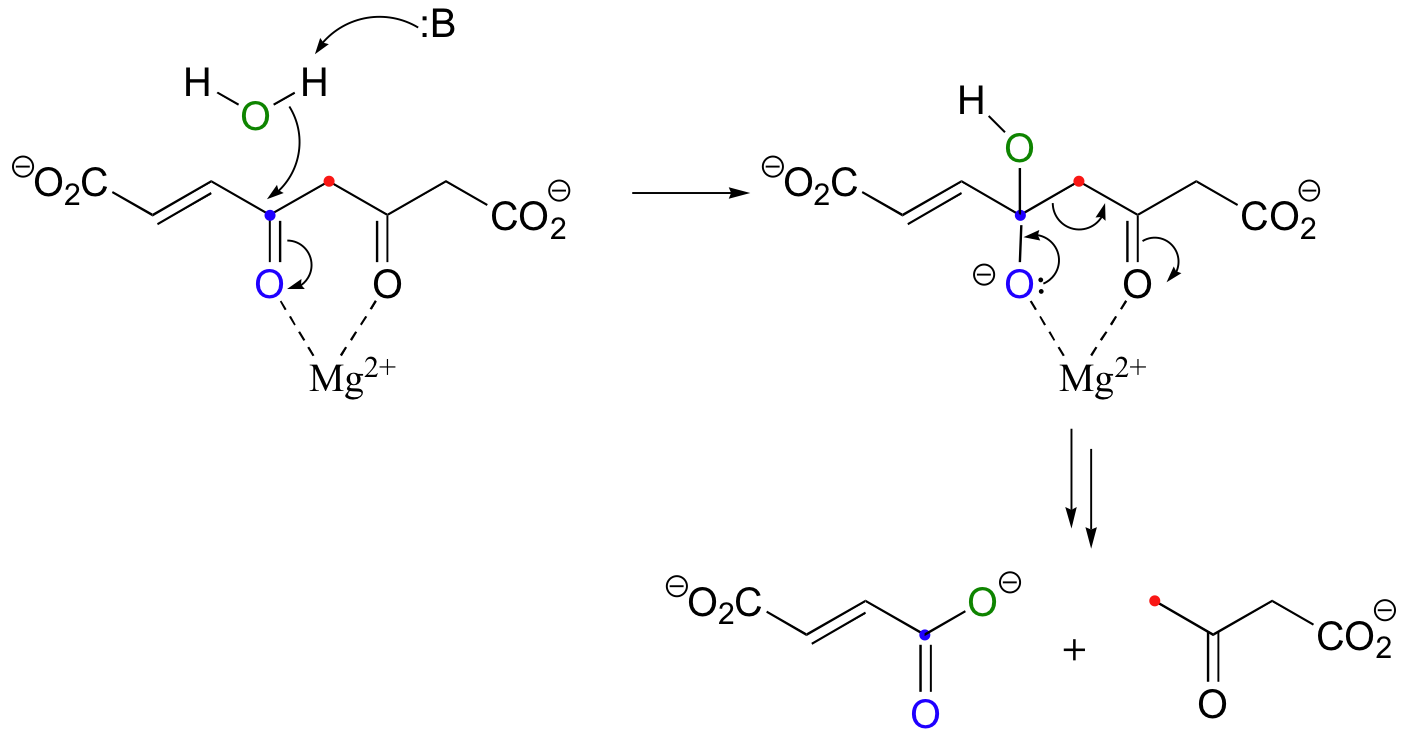
In the mechanism above, the final enolate-to-keto protonation step is not shown. Notice that in this reaction, negative charges on both oxygens are stabilized by interaction with a magnesium ion.
13.4C: Enolates as nucleophiles in SN2 displacements
Finally, we will now consider a twist on the Claisen reaction type, in which the essential substitution mechanism is not an acyl substitution at a carbonyl carbon but an associative nucleophilic substitution (SN2) event at a tetrahedral carbon center. In section 9.1A we saw some examples of SN2 methylation reactions using S-adenosyl methionine (SAM). In those examples, the nucleophile was either an alcohol oxygen or an amine nitrogen. In the following example (a reaction in the biosynthesis of the antibiotic indolmycin) the nucleophile is the alpha-carbon of an enol intermediate.
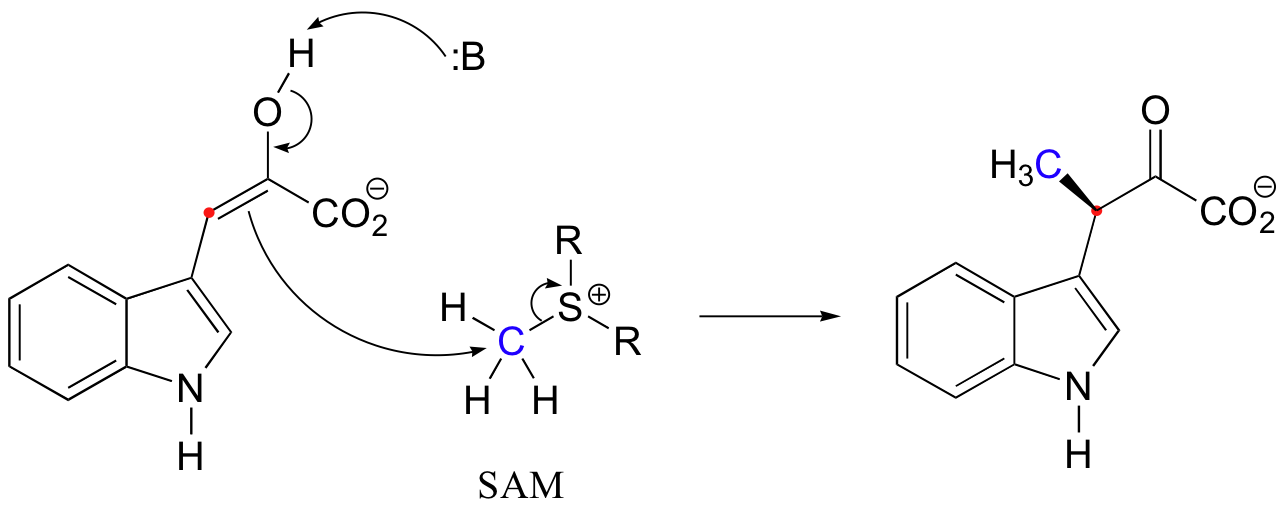
Isotopic labeling studies (using 1H, 2H and 3H at the electrophilic methyl carbon of SAM to make it chiral) indicate that, as expected, the reaction proceeds with inversion of configuration at the methyl carbon (J. Am. Chem. Soc. 1977, 99, 273).


