21.5: Los usos de los radioisótopos
- Page ID
- 1982
Habilidades para desarrollar
-
Liste las aplicaciones comunes de los isótopos radiactivos
Los isótopos radiactivos tienen las mismas propiedades químicas que los isótopos estables del mismo elemento, pero emiten radiación, que se puede detectar. Si reemplazamos uno (o más) átomo(s) con radioisótopos en un compuesto, podemos rastrearlos monitoreando sus emisiones radioactivas. Este tipo de compuesto se llama el marcador radiactivo. Los radioisótopos se usan para seguir los caminos de las reacciones bioquímicas o para determinar cómo se distribuye una sustancia dentro de un organismo. Los trazadores radiactivos también se usan en muchas aplicaciones médicas, incluidos el diagnóstico y el tratamiento. Se usan para medir el desgaste del motor, analizar la formación geológica alrededor de los pozos de petróleo y mucho más.
Los radioisótopos han revolucionado la práctica médica, donde se usan extensivamente. En los Estados Unidos se realizan anualmente más de 10 millones de procedimientos de medicina nuclear y más de 100 millones de pruebas de medicina nuclear. Cuatro ejemplos típicos de trazadores radiactivos utilizados en la medicina son tecnecio-99 \(\ce{(^{99}_{43}Tc)}\), talio-201 \(\ce{(^{201}_{81}Tl)}\), yodo-131 \(\ce{(^{131}_{53}I)}\) y sodio-24 \(\ce{(^{24}_{11}Na)}\). Los tejidos dañados en el corazón, el hígado y los pulmones absorben preferentemente ciertos compuestos de tecnecio-99. Cuando se inyecta, se puede determinar la ubicación del compuesto de tecnecio y, por tanto, el tejido dañado, detectando los rayos γ emitidos por el isótopo Tc-99. El talio-201 (Figura \(\PageIndex{1}\)) se concentra en el tejido cardíaco sano, por eso los dos isótopos, Tc-99 y Tl-201, se usan juntos para estudiar el tejido cardíaco. El yodo-131 se concentra en la glándula tiroides, el hígado y algunas partes del cerebro. Por eso, se puede usar para controlar el bocio y tratar afecciones de la tiroides, como la enfermedad de Grave, así como los tumores hepáticos y cerebrales. Las soluciones salinas que contienen compuestos de sodio-24 se inyectan en el torrente sanguíneo para ayudar a localizar las obstrucciones en el flujo sanguíneo.
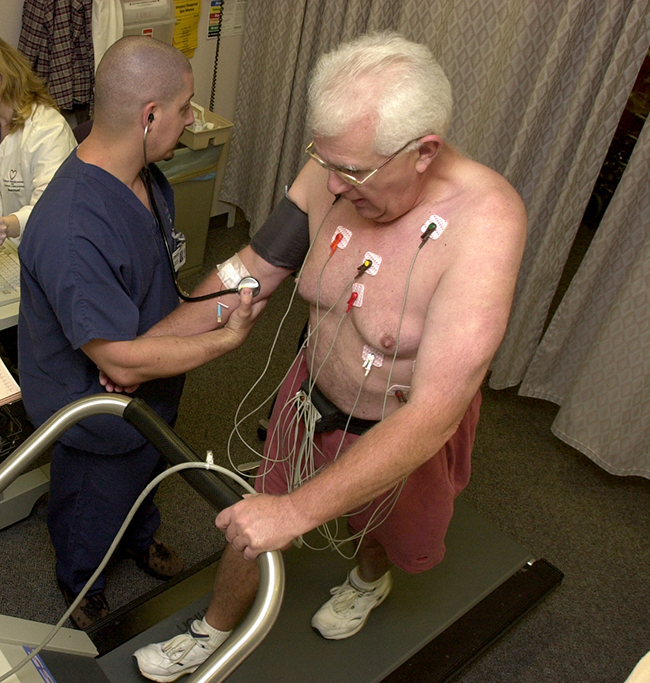
Figura \(\PageIndex{1}\): La administración de talio-201 a un paciente y luego la realización de una prueba de esfuerzo, le da a los profesionales médicos la oportunidad de analizar visualmente la función cardíaca y el flujo sanguíneo. (crédito: modificación del trabajo de “Blue0ctane”/Wikimedia Commons)
Los radioisótopos usados en la medicina suelen tener una vida media corta; por ejemplo, el ubicuo Tc-99m tiene una vida media de 6.01 horas. Esto hace que el Tc-99m sea esencialmente imposible de almacenar y extremadamente costoso de transportar, por eso se fabrica en el sitio. Los hospitales y otras instalaciones médicas usan el Mo-99 (que se extrae principalmente de los productos de fisión del U-235) para generar Tc-99. El Mo-99 pasa por una desintegración β con una vida media de 66 horas, y luego el Tc-99 se extrae químicamente (Figura \(\PageIndex{2}\)). El nucleido padre Mo-99 es parte de un ion molibdato, \(\ce{MoO4^2-}\); cuando decae, forma el ion pertecnetato, \(\ce{TcO4-}\). Estos dos iones que están solubles en el agua se separan usando la cromatografía en columna, con el ión molibdato de carga superior adsorbiéndose sobre la alúmina en la columna, y pasando el ión pertecnetato con una carga más baja a través de la columna en la solución. Unos pocos microgramos de Mo-99 pueden producir suficiente Tc-99 para realizar hasta 10,000 pruebas.
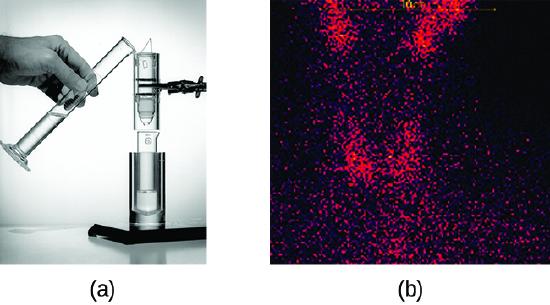
Figura \(\PageIndex{2}\):(a) El primer generador de Tc-99m (alrededor de 1958) se usa para separar el Tc-99 del Mo-99. \(\Ce{MoO4^2-}\) es retenido por la matriz en la columna, mientras que \(\ce{TcO4-}\) pasa y esta coleccionado. (b) Se usó el Tc-99 en la exploración del cuello de un paciente con la enfermedad de Graves. El escaneo muestra la ubicación de altas concentraciones de Tc-99. (crédito a: modificación del trabajo por parte del Departamento de Energía; crédito b: modificación del trabajo por “MBq”/Wikimedia Commons)
También se pueden usar los radioisótopos como tratamiento, típicamente en dosis más altas que como un trazador. La radioterapia es el uso de radiación de alta energía para dañar el ADN de las células cancerosas, lo que las mata o evita que se dividan (Figura \(\PageIndex{3}\)). Un paciente con cáncer puede recibir radioterapia de haz externo administrada por una máquina fuera del cuerpo o radioterapia interna (braquiterapia) a partir de una sustancia radiactiva que se ha introducido en el cuerpo. Tenga en cuenta que la quimioterapia es similar a la radioterapia interna en que el tratamiento del cáncer se inyecta en el cuerpo, pero difiere en que la quimioterapia usa sustancias químicas en lugar de radiactivas para destruir las células cancerosas.
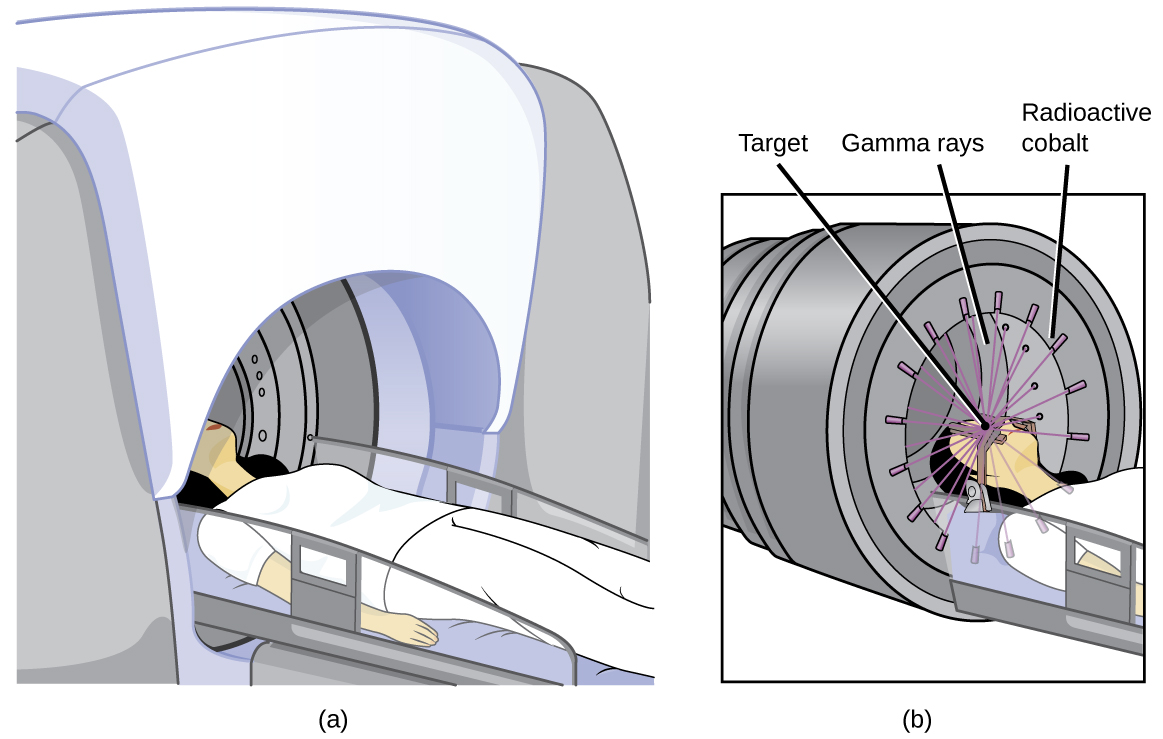
Figura \(\PageIndex{3}\): La caricatura en (a) muestra una máquina de cobalto-60 usada en el tratamiento del cáncer. El diagrama en (b) muestra cómo el pórtico de la máquina Co-60 gira a través de un arco, enfocando la radiación en la región objetivo (tumor) y minimizando la cantidad de radiación que pasa a través de las regiones cercanas.
El cobalto-60 es un radioisótopo sintético producido por la activación neutrónica de Co-59, que luego pasa por la desintegración β para formar Ni-60, junto con la emisión de radiación γ. El proceso general es:
\[\ce{^{59}_{27}Co + ^1_0n⟶ ^{60}_{27}Co⟶ ^{60}_{28}Ni + ^0_{−1}β + 2^0_0γ}\]
El proceso de decaimiento general para esto se muestra gráficamente en la Figura \(\PageIndex{4}\).
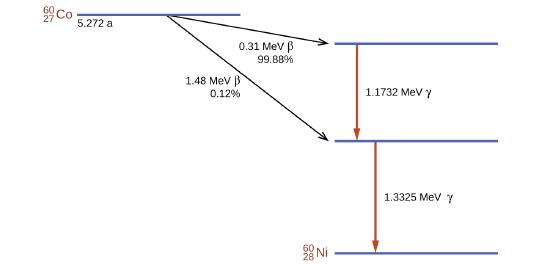
Figura \(\PageIndex{4}\): El Co-60 pasa por una serie de desintegraciones radiactivas. Las emisiones γ se usan para radioterapia.
Los radioisótopos se usan de diversas maneras para estudiar los mecanismos de reacciones químicas en las plantas y los animales. Estos incluyen etiquetado de fertilizantes en estudios de absorción de nutrientes por plantas y crecimiento de cultivos, investigaciones de procesos digestivos y de producción de leche en vacas y estudios sobre el crecimiento y metabolismo de los animales y las plantas.
Por ejemplo, el radioisótopo C-14 se usó para dilucidar los detalles de cómo se produce la fotosíntesis. La reacción general es:
\[\ce{6CO2}(g)+\ce{6H2O}(l)⟶\ce{C6H12O6}(s)+\ce{6O2}(g),\]
pero el proceso es mucho más complejo y pasa por una serie de pasos en los que se producen varios compuestos orgánicos. En los estudios de la vía de esta reacción, las plantas fueron expuestas a CO2 que contenía una alta concentración de \(\ce{^{14}_6C}\). A intervalos regulares, se analizaron las plantas para determinar qué compuestos orgánicos contenían carbono-14 y qué cantidad de cada compuesto estaba presente. A partir de la secuencia de tiempo en la que aparecieron los compuestos y la cantidad de cada presente en intervalos de tiempo dados, los científicos aprendieron más sobre la vía de la reacción.
Las aplicaciones comerciales de los materiales radiactivos son igualmente diversas (Figura \(\PageIndex{5}\)). Incluyen determinar el espesor de las películas y láminas de metal delgadas aprovechando del poder de la penetración de varios tipos de radiación. Los defectos en los metales utilizados con fines estructurales se pueden detectar usando los rayos gamma de alta energía de cobalto-60 de una manera similar a la forma en que se usan los rayos X para examinar el cuerpo humano. En una forma de control de plagas, las moscas se controlan por esterilizando las moscas macho con radiación γ para que las hembras que se reproducen con ellas no produzcan descendencia. Muchos alimentos se conservan usando la radiación que mata los microorganismos que hacen que se echen a perder.

Figura \(\PageIndex{5}\): Los usos comerciales comunes de la radiación incluyen (a) el examen de los rayos X del equipaje en un aeropuerto y (b) la conservación de los alimentos. (crédito a: modificación del trabajo por parte del Departamento de la Marina; crédito b: modificación del trabajo por parte del Departamento de Agricultura de EE. UU.)
El americio-241, un emisor α con una vida media de 458 años, se usa en pequeñas cantidades en detectores de humo de tipo ionización (Figura \(\PageIndex{6}\)). Las emisiones α de Am-241 ionizan el aire entre dos placas de electrodos en la cámara de ionización. Una batería suministra un potencial que provoca el movimiento de los iones, haciendo así una pequeña corriente eléctrica. Cuando el humo ingresa a la cámara, se impide el movimiento de los iones, lo que reduce la conductividad del aire. Esto provoca una caída en la corriente, lo que activa una alarma.
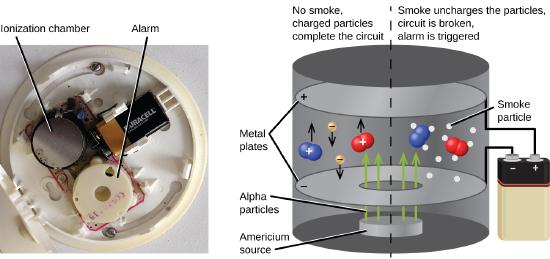
Figura \(\PageIndex{6}\): Dentro de un detector de humo, Am-241 emite partículas α que ionizan el aire, haciendo una pequeña corriente eléctrica. Durante un incendio, las partículas de humo impiden el flujo de iones, reduciendo la corriente y activando una alarma. (crédito a: modificación del trabajo de “Muffet” / Wikimedia Commons)
Resumen
Los compuestos conocidos como los trazadores radiactivos se pueden usar para seguir las reacciones, rastrear la distribución de una sustancia, diagnosticar y tratar afecciones médicas y mucho más. Otras sustancias radiactivas son útiles para controlar las plagas, visualizar las estructuras, proporcionar las advertencias de incendio y para muchas otras aplicaciones. Cada año en los EE.UU. se realizan cientos de millones de pruebas y procedimientos de medicina nuclear que usan una extensa variedad de radioisótopos con vidas medias relativamente cortas. La mayoría de estos radioisótopos tienen vidas medias relativamente cortas; algunos son lo suficientemente cortos como para que el radioisótopo se deba fabricar in situ en las instalaciones médicas. La radioterapia usa la radiación de alta energía para destruir las células cancerosas al dañar su ADN. La radiación utilizada para este tratamiento se puede administrar externa o internamente.
Glosario
- la quimioterapia
- similar a la radioterapia interna, pero se introducen en el cuerpo sustancias químicas en lugar de radiactivas para destruir las células cancerosas
- radioterapia de haz externo
- radiación emitida por una máquina fuera del cuerpo
- radioterapia interna
- (también, braquiterapia) radiación de una sustancia radiactiva que se introduce en el cuerpo para destruir las células cancerosas
- radioterapia
- uso de radiación de alta energía para dañar el ADN de las células cancerosas, lo que las mata o evita que se dividan
- trazador radiactivo
- (también, etiqueta radiactiva) radioisótopo usado para rastrear o seguir una sustancia mediante el control de sus emisiones radiactivas
Contribuyentes y atribuciones
Paul Flowers (Universidad de Carolina del Norte - Pembroke), Klaus Theopold (Universidad de Delaware) y Richard Langley (Stephen F. Austin Universidad del Estado) con autores contribuyentes. Contenido del libro de texto producido por la Universidad de OpenStax tiene licencia de Atribución de Creative Commons Licencia 4.0 licencia. Descarge gratis en http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110)."
Ana Martinez (amartinez02@saintmarys.edu) contribuyó a la traducción de este texto.


