17.3: Reacciones en cadena de radicales
- Page ID
- 2457
17.2A: The three phases of radical chain reactions
Because of their high reactivity, free radicals have the potential to be both extremely powerful chemical tools and extremely harmful contaminants. Much of the power of free radical species stems from the natural tendency of radical processes to occur in a chain reaction fashion. Radical chain reactions have three distinct phases: initiation, propagation, and termination.
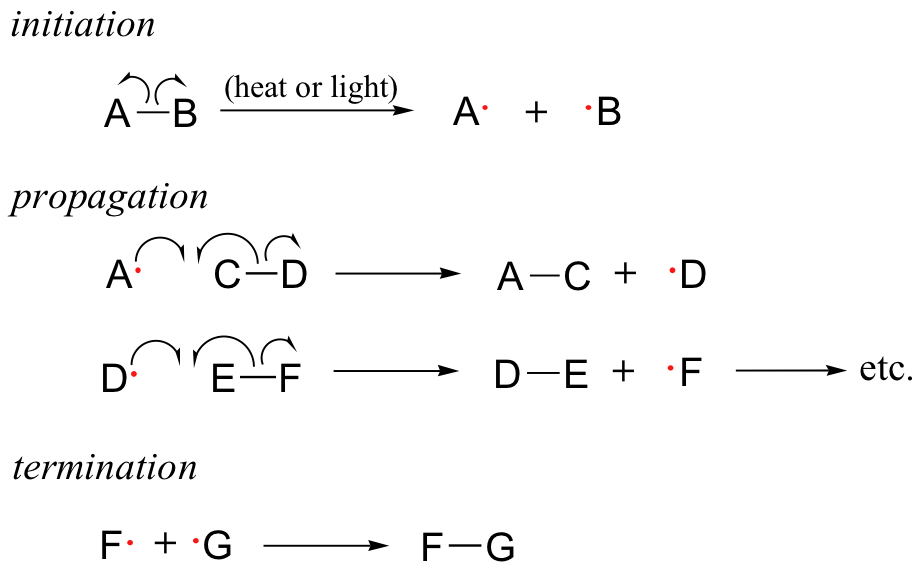
The initiation phase describes the step that initially creates a radical species. In most cases, this is a homolytic cleavage event, and takes place very rarely due to the high energy barriers involved. Often the influence of heat, UV radiation, or a metal-containing catalyst is necessary to overcome the energy barrier.
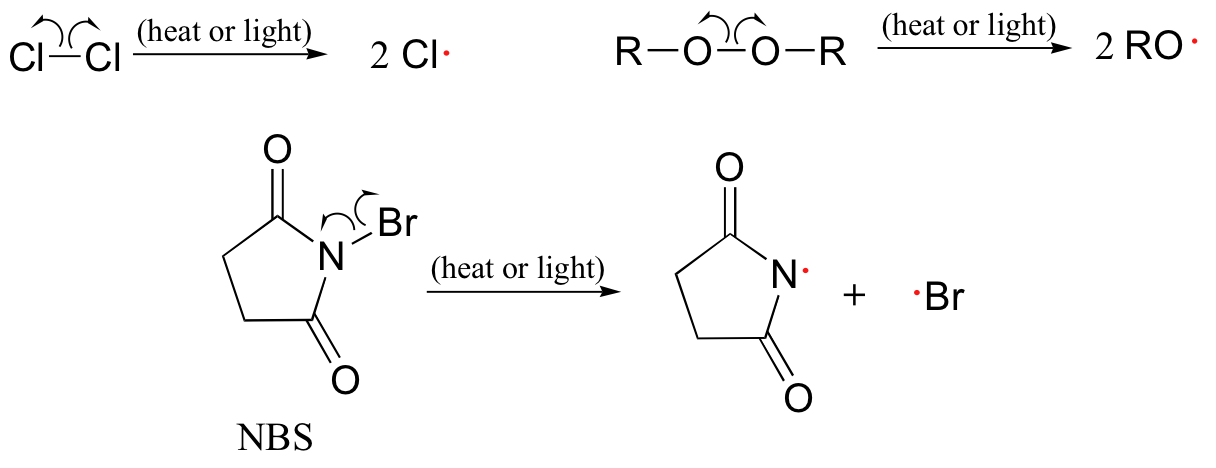
Molecular chlorine and bromine will both undergo homolytic cleavage to form radicals when subjected to heat or light. Other functional groups which also tend to form radicals when exposed to heat or light are chlorofluorocarbons, peroxides, and the halogenated amide N-bromosuccinimide (NBS).
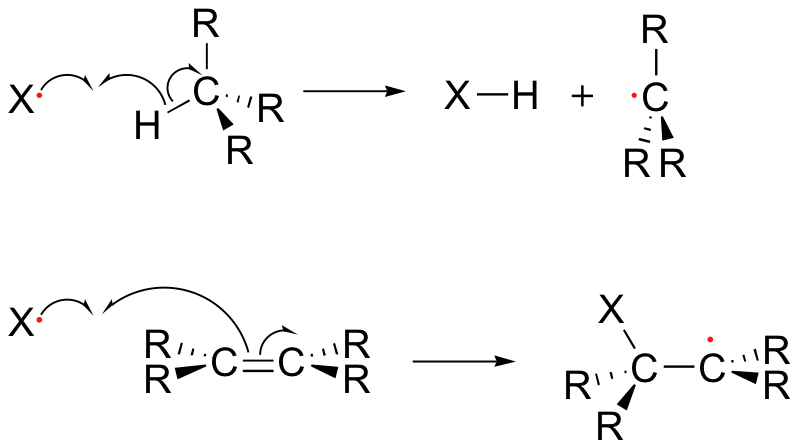
The propagation phase describes the 'chain' part of chain reactions. Once a reactive free radical is generated, it can react with stable molecules to form new free radicals. These new free radicals go on to generate yet more free radicals, and so on. Propagation steps often involve hydrogen abstraction or addition of the radical to double bonds.
Chain termination occurs when two free radical species react with each other to form a stable, non-radical adduct. Although this is a very thermodynamically downhill event, it is also very rare due to the low concentration of radical species and the small likelihood of two radicals colliding with one another. In other words, the Gibbs free energy barrier is very high for this reaction, mostly due to entropic rather than enthalpic considerations. The active sites of enzymes, of course, can evolve to overcome this entropic barrier by positioning two radical intermediates adjacent to one another.
17.2B: Radical halogenation in the lab
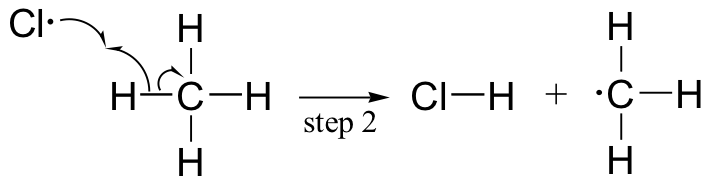
The chlorination of an alkane provides a simple example of a free radical chain reaction. In the initiation phase, a chlorine molecule undergoes homolytic cleavage after absorbing energy from light:
![]()
The chlorine radical then abstracts a hydrogen, leading to an alkyl radical (step 2), which reacts with a second chlorine molecule (step 3) to form the chloroalkane product plus chlorine radical, which then returns to repeat step 2.
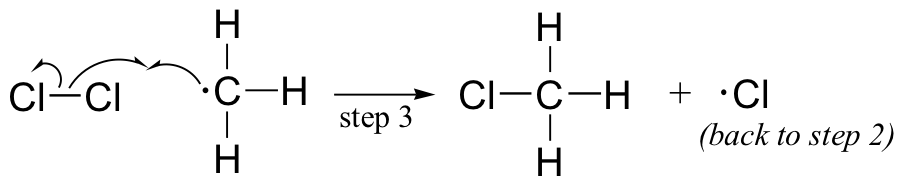
Likely chain termination steps are the condensation of two alkyl radical intermediates or condensation of an alkane radical with a chlorine radical.
Alkane halogenation reactions exhibit a degree of regiospecificity: if 2-methylbutane is subjected to a limiting amount of chlorine, for example, chlorination takes place fastest at the tertiary carbon.
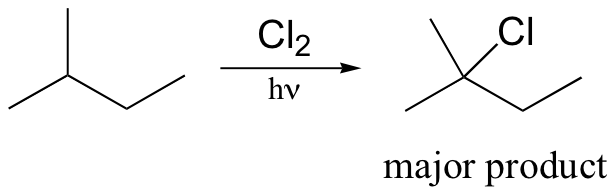
This is because the tertiary radical intermediate is more stable than the secondary radical intermediate that results from abstraction of the proton on carbon #3, and of course both are more stable than a primary radical intermediate. Recall that the Hammond postulate (section 6.2, section 15.2B) tells us that a lower-energy intermediate implies a lower-energy transition state, and thus a faster reaction.
Unfortunately, chloroalkanes will readily undergo further chlorination resulting in polychlorinated products, so this is not generally a terribly useful reaction from a synthetic standpoint.
Alkanes can be brominated by a similar reaction. The regiochemical trends are the same as for chlorination, but significantly more pronounced (in other words, bromination is more regioselective). This is because hydrogen abstraction by bromine radical is much less exergonic than by chorine radical – and this in turn means that the transition state for abstraction by bromine resembles the resulting intermediate more closely than the transition state for abstraction by chlorine resembles its intermediate.
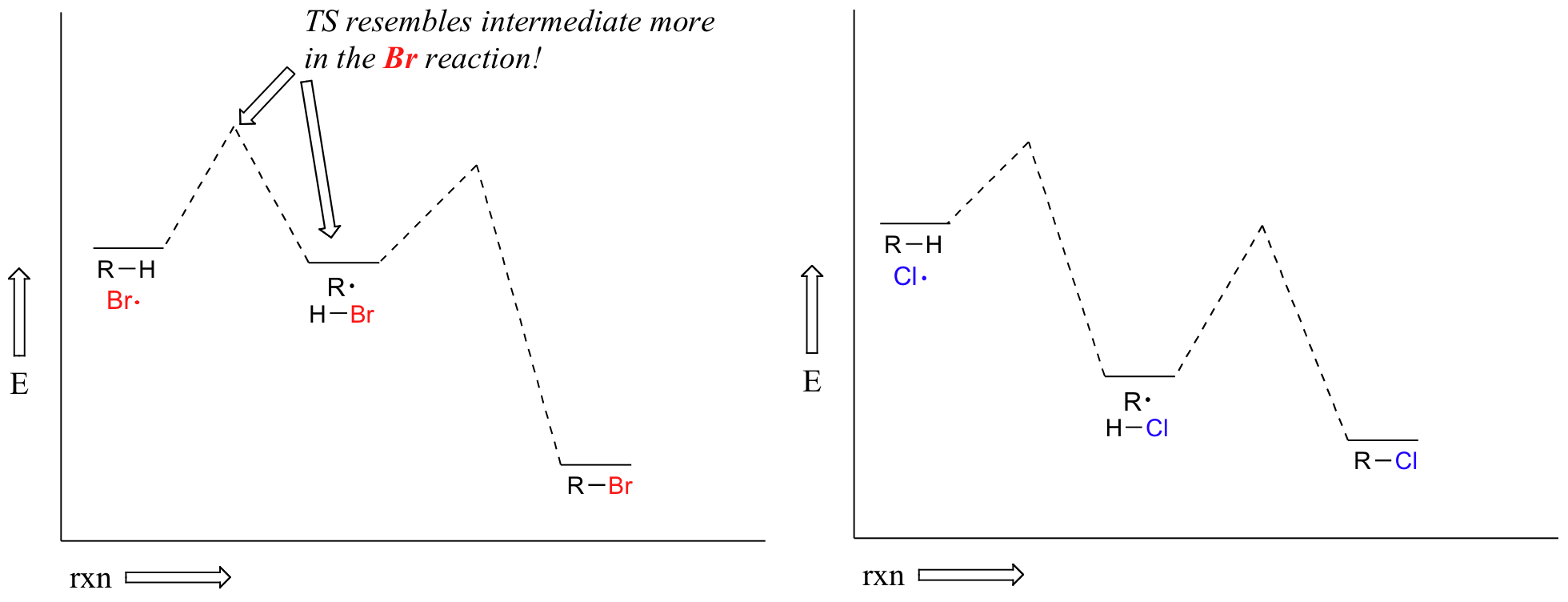
Another way of saying the same thing is that the bromination transition state has more ‘radical character’ than the chlorination transition state. Trends in radical stability thus have a greater influence on the speed of hydrogen abstraction.
Alkenes and alkylbenzene can be halogenated with high regiospecificity using N-bromosuccinimide (NBS) in the presence of light or a radical initiator such as benzoyl peroxide. Bromination takes place specifically at the allylic position of alkenes and at the benzylic position of alkylbenzene (recall from section 17.1A that allylic and benzylic radicals are stabilize by resonance).
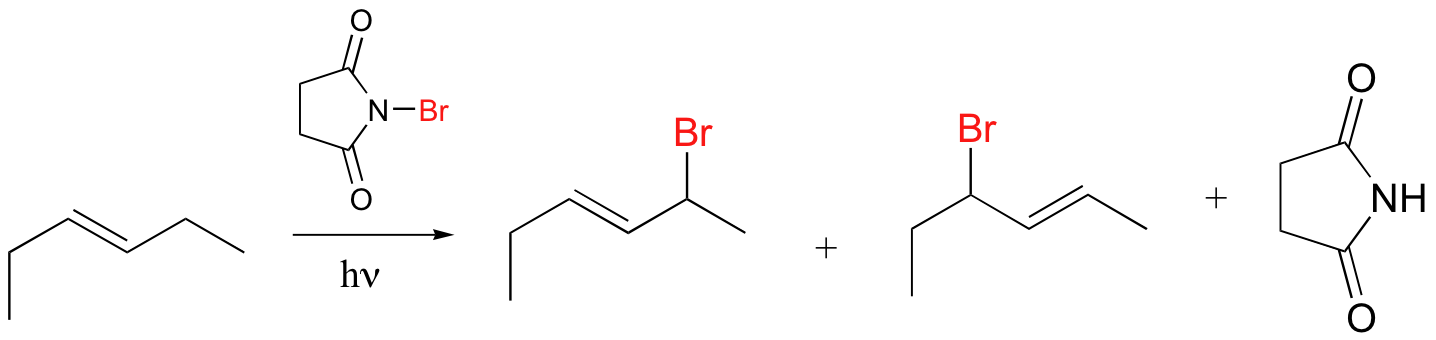
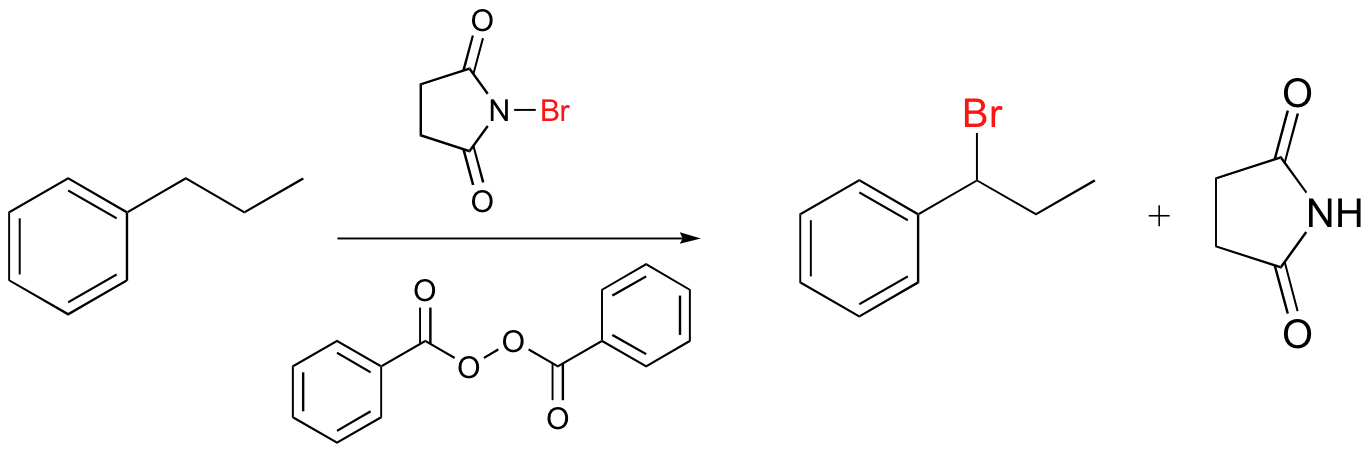
A mechanism for allylic bromination by NBS is shown below:
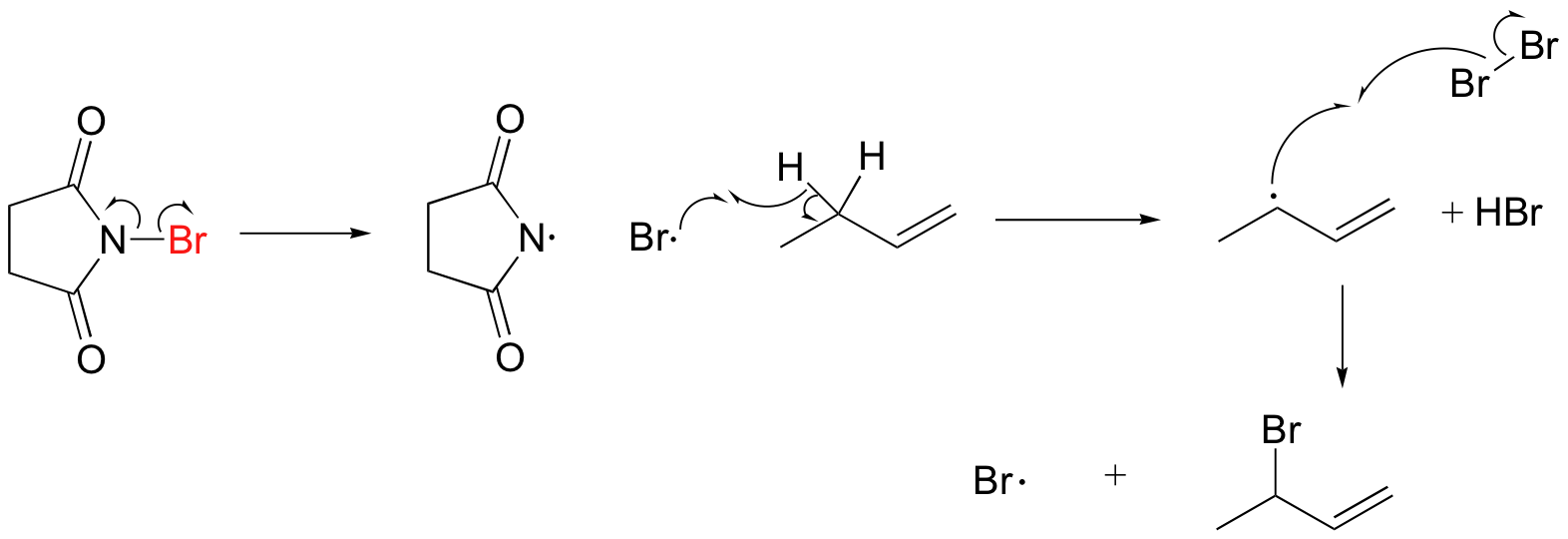
The Br2 in this process is formed in a side reaction between HBr and NBS (not shown).
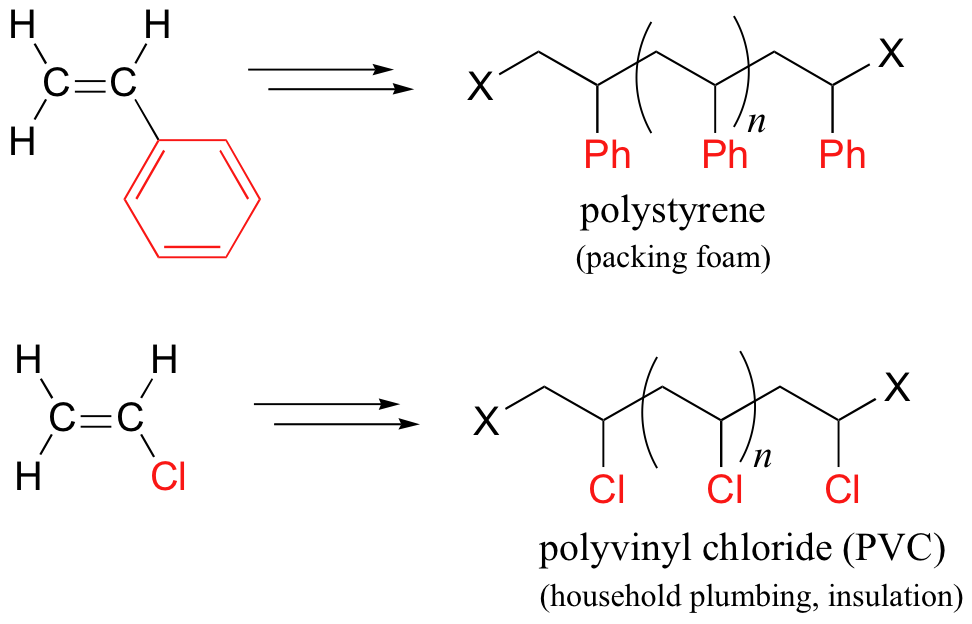
17.2C: Useful polymers formed by nonenzymatic radical chain reactions
Many household polymeric materials with which you are probably familiar are made with a radical chain reaction process. Polyethylene (PET), the plastic material used to make soft drink bottles and many other kinds of packaging, is produced by the radical polymerization of ethylene (ethene in IUPAC nomenclature). A radical initiator such as benzoyl peroxide undergoes homolytic cleavage when subjected to high temperatures.
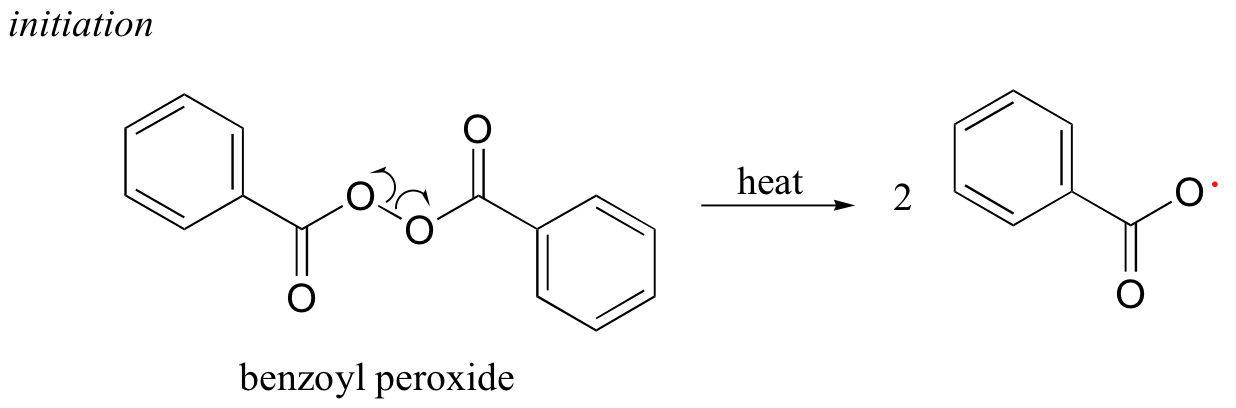
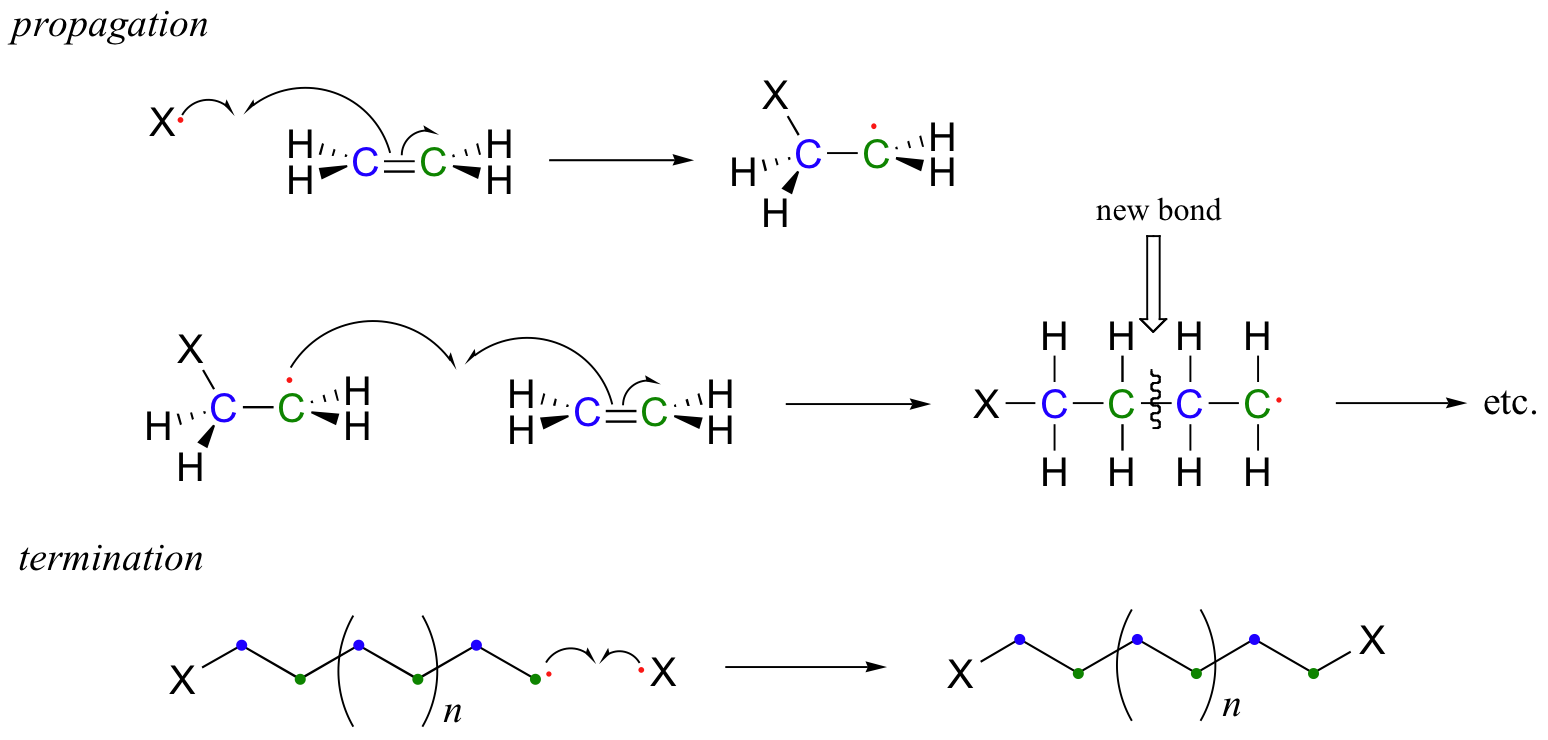
In the propagation phase, the benzoyl radical (X• in the figure below) adds to the double bond of ethylene, generating a new organic radical. Successive ethylene molecules add to the growing polymer, until termination occurs when two radicals happen to collide. In the figure below, the growing PET polymer is terminated by a benzoyl radical, but in an alternative termination step two growing PET radicals could condense.
Other small substituted alkene monomers polymerize in a similar fashion to form familiar polymer materials. Two examples are given below.
17.2D: Destruction of the ozone layer by CFC radicals
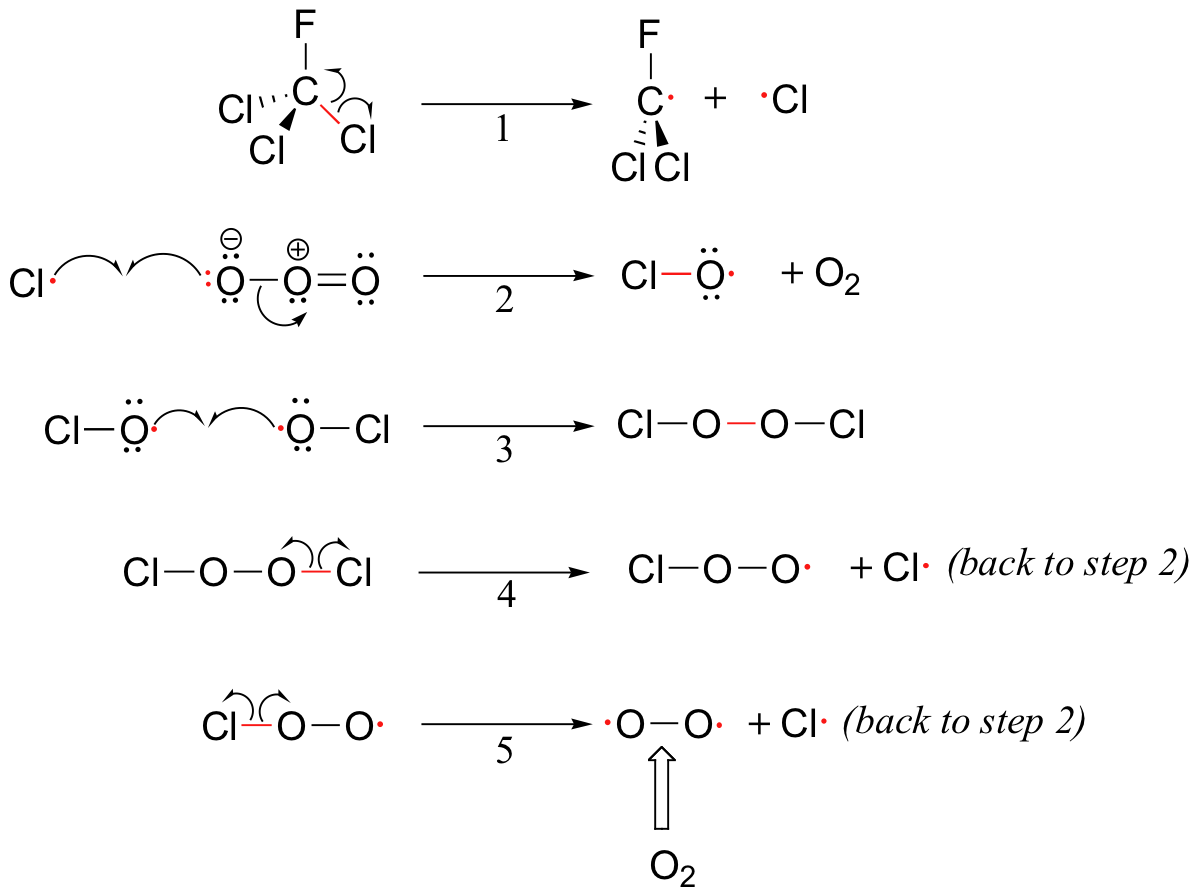
The high reactivity of free radicals and the multiplicative nature of radical chain reactions can be useful in the synthesis of materials such as polyethylene plastic - but these same factors can also result in dangerous consequences. You are probably aware of the danger posed to the earth's protective stratospheric ozone layer by the use of chlorofluorocarbons (CFCs) as refrigerants and propellants in aerosol spray cans. Freon-11, or CFCl3, is a typical CFC that was widely used until fairly recently. It can take months or years for a CFC molecule to drift up into the stratosphere from the surface of the earth, and of course the concentration of CFCs at this altitude is very low. Ozone, on the other hand, is continually being formed in the stratosphere. Why all the concern, then, about destruction of the ozone layer - how could such a small amount of CFCs possibly do significant damage? The problem lies in the fact that the process by which ozone is destroyed is a chain reaction, so that a single CFC molecule can initiate the destruction of many ozone molecules before a chain termination event occurs.
Although there are several different processes by which the ozone destruction process might occur, the most important is believed to be the chain reaction shown below.
To address the problem of ozone destruction, scientists are developing new organohalogen refrigerant compounds that are less stable than the older CFCs like Freon-11, in the hope that the new compounds will break down in the lower atmosphere before they reach an altitude where they can harm the ozone layer. Most of the new compounds contain carbon-hydrogen bonds, which are subject to homolytic cleavage initiated by hydroxide radicals present in the lower atmosphere.
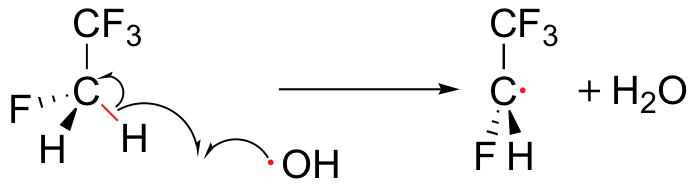
This degradation occurs before the refrigerant molecules have a chance to drift up to the stratosphere where the ozone plays its important protective role. The degradation products are quite unstable and quickly degrade further, by a variety of mechanisms, into relatively harmless by-products. The hydroxide radical is sometimes referred to as an atmospheric 'detergent' due to its ability to degrade refrigerants and other volatile organic pollutants which have escaped into the atmosphere.
17.2E: Harmful radical species in cells and natural antioxidants
While the high reactivity of the hydroxide radical is a beneficial trait in the atmosphere, it is a harmful trait when the same hydroxide radical is present in a living cell. Hydroxide radical and other reactive oxygen species (ROS) such as superoxide (O2-) and peroxide (O2 2-) are continuously produced as minor side-products in the reduction of O2 to H2O in respiration.
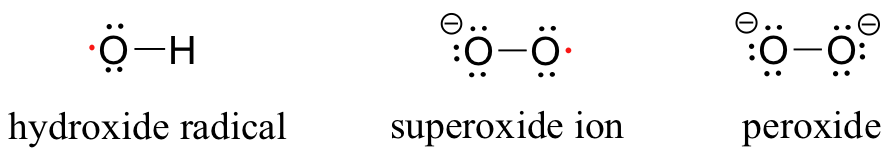
The ROS are highly reactive oxidizing agents, capable of inflicting damage to DNA, proteins, and the lipids of cell membranes - they are thought to play a major role in the natural aging process. Hydroxide radical, for example, will initiate a radical chain reaction with the hydrocarbon part of an unsaturated membrane lipid molecule that results in the formation of lipid hydroperoxide.
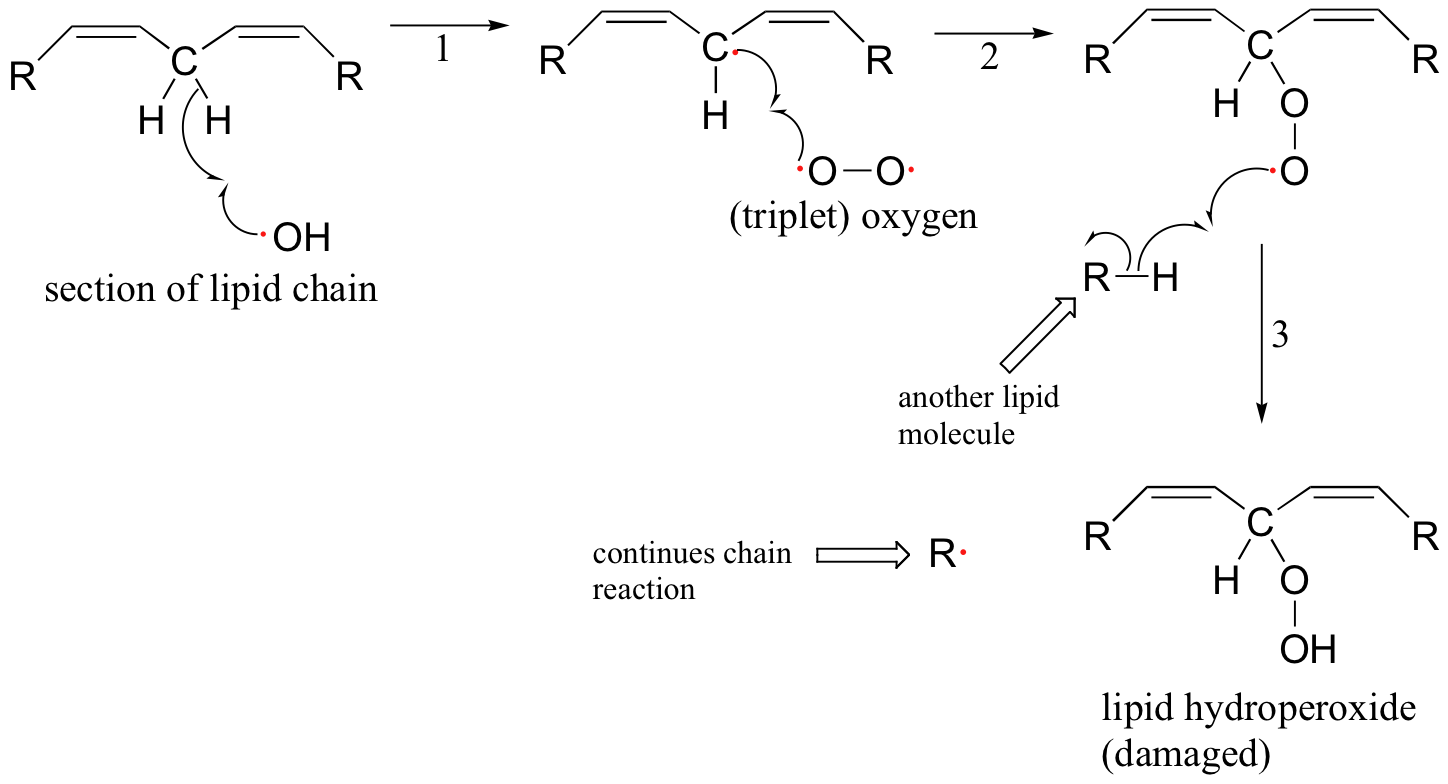
The allylic lipid radical formed as the result of homolytic hydrogen abstraction by hydroxide radical (step 1 above) reacts with one of the unpaired electrons in triplet oxygen (step 2) forming a peroxy radical. This radical species in turn abstracts a hydrogen from another lipid molecule (step 3), thus propagating the chain.
Superoxide anion and peroxide are converted to molecular oxygen and water by protective enzymes called superoxide dismutase and catalase, but no such enzymatic defense exists against the hydroxide radical. Antioxidant compounds, also known as 'free radical scavengers', serve to protect cells from the oxidative effects of reactive oxygen species and other harmful radical intermediates. Simply put, a free radical scavenger is a molecule that reacts with a high energy free radical species (like the lipid peroxide radical formed in step 2 of the figure above) in a radical chain propagation step, forming a more stable radical species which can be metabolized in some way before further damage is done to cell constituents.
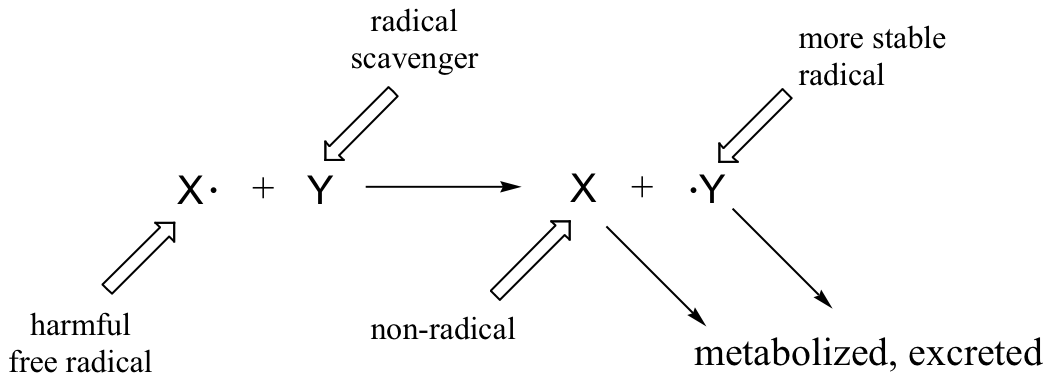
One important antioxidant that you are no doubt familiar with is ascorbic acid, or vitamin C. Here is how ascorbate (the deprotonated form of ascorbic acid) acts as a free radical scavenger: when it encounters a free radical (denoted X• in the figure below), ascorbate donates a single electron to become ascorbyl radical.
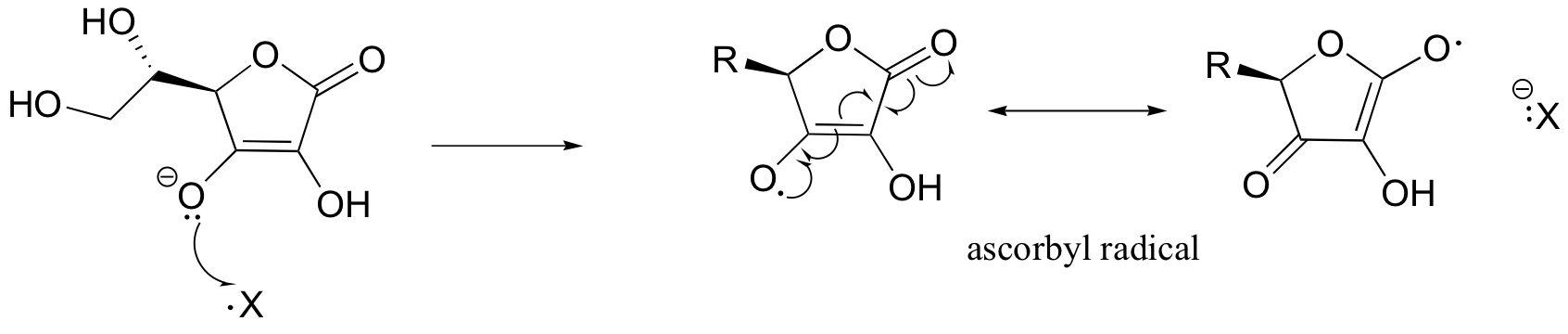
Ascorbyl radical is significantly more stable than most other radical species due to resonance delocalization. The end result of this first step is that a very reactive, potentially harmful radical (X•) has been 'quenched', and replaced by a much less reactive (and thus less harmful) ascorbyl radical.
Next, the ascorbyl radical can donate a second electron to another potentially harmful radical species, resulting in the formation of dehydroascorbate, the oxidized form of ascorbate (it is actually the cyclic hemiacetal, hydrated form of dehydroascorbic acid that is thought to be prevalent in physiological conditions).
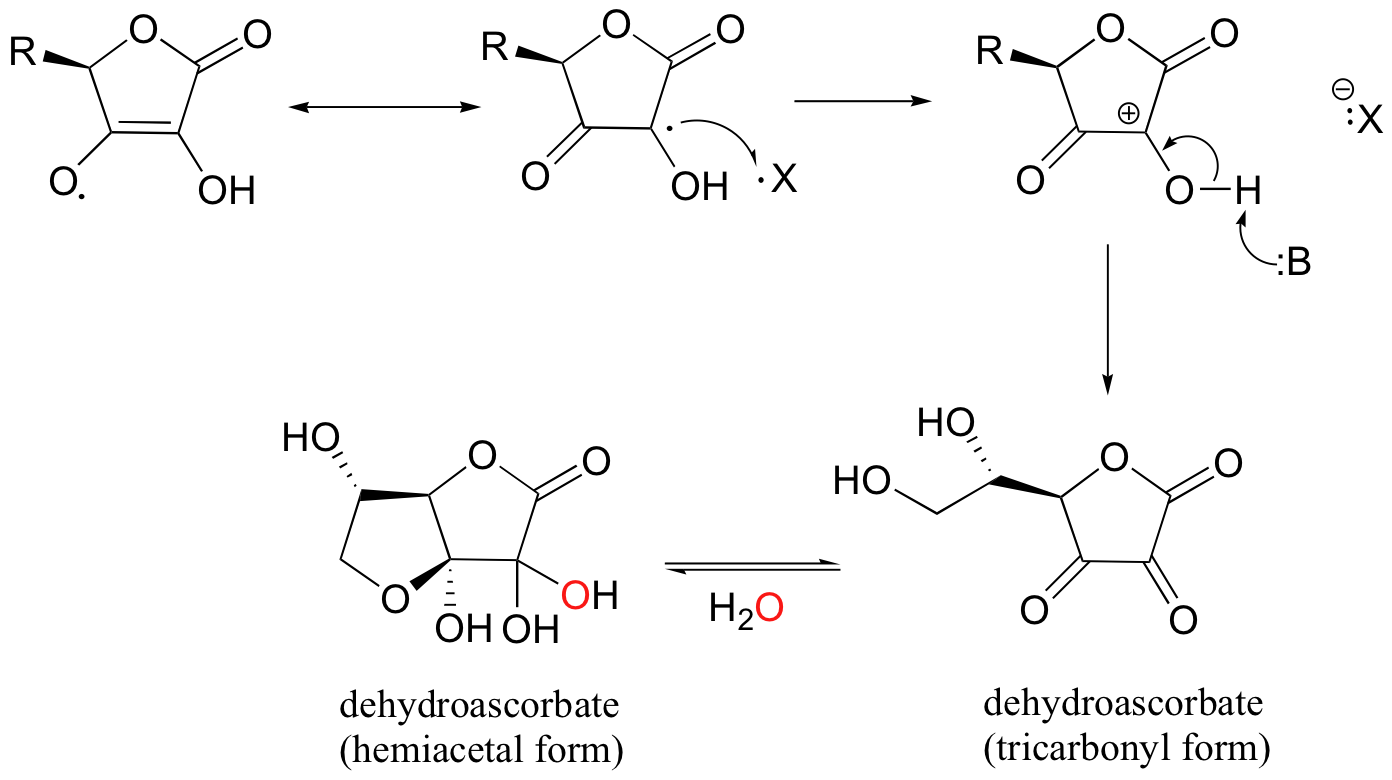
One ascorbate molecule is thus potentially able to scavenge two harmful radical species.
Dehydroascorbate is subsequently either broken down and excreted, or else recycled (reduced) back to ascorbate. This can happen either in a direct, enzyme-free reaction with glutathione, or through the action of a specific glutathione/NADH-dependant reductase enzyme. You were invited to propose a likely mechanism for the enzyme-free reaction in problem 16.14.
You are probably aware that many fruits and vegetables contain natural antioxidant compounds that are thought to be beneficial to our health. Most of these are polyphenols (thus named because they contain multiple phenol groups). Apigenin, for example, is found in parsley and celery, while the skins of grapes used to produce red wine are particularly rich in resveratrol as well as many other polyphenols.
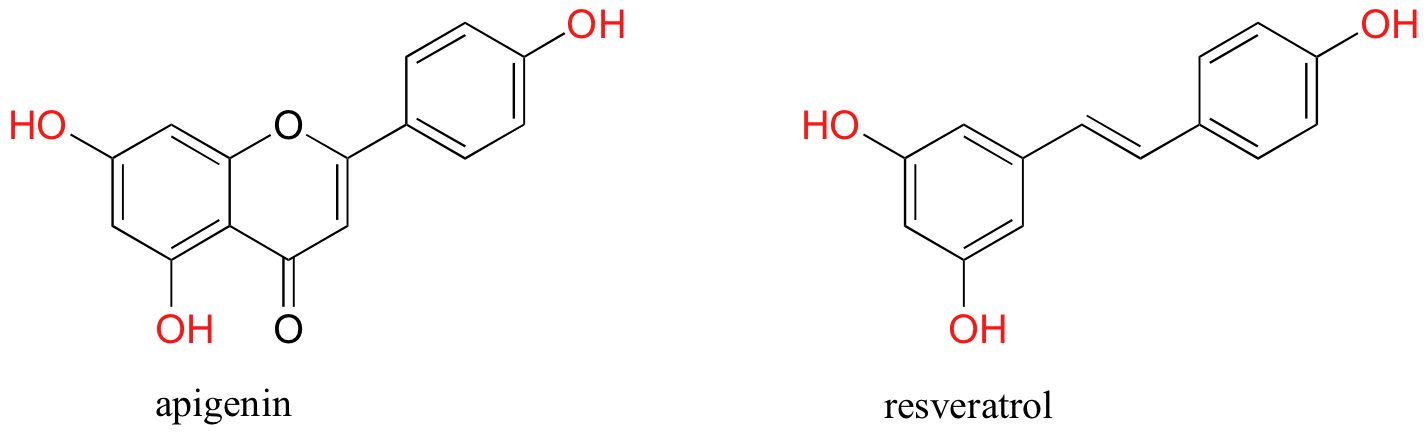
While little is known about exactly how these polyphenols exert their antioxidant effect, it is likely that they, like ascorbic acid, act as radical scavengers. The stability of a polyphenol radical can be explained, as you might expect, by the concept of resonance delocalization.


