Propiedades del Agua
- Page ID
- 77731
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)| Densidad del agua (kg/m 3) a diferentes temperaturas (°C) | |
|---|---|
| Temperatura 1 | Densidad |
| 0 | 999.8395 |
| 4 | 999.9720 (densidad máxima) |
| 10 | 999.7026 |
| 15 | 999.1026 |
| 20 | 998.2071 |
| 22 | 997.7735 |
| 25 | 997.0479 |
| 30 | 995.6502 |
| 40 | 992.2 |
| 60 | 983.2 |
| 80 | 971.8 |
| 100 | 958.4 |
| Presión de Vapor de Agua a Diferentes Temperaturas (°C) | ||
|---|---|---|
| Temperatura | Presión de vapor (torr) | Presión de Vapor (Pa) |
| 0 | 4.6 | 613.2812 |
| 4 | 6.1 | 813.2642 |
| 10 | 9.2 | 1226.562 |
| 15 | 12.8 | 1706.522 |
| 20 | 17.5 | 2333.135 |
| 22 | 19.8 | 2639.776 |
| 25 | 23.8 | 3173.064 |
| 30 | 31.8 | 4239.64 |
| 35 | 42.2 | 5626.188 |
| 40 | 55.3 | 7372.707 |
| 45 | 71.9 | 9585.852 |
| 50 | 92.5 | 12332.29 |
| 55 | 118.0 | 15732 |
| 60 | 149.4 | 19918.31 |
| 65 | 187.5 | 24997.88 |
| 70 | 233.7 | 31157.35 |
| 75 | 289.1 | 38543.39 |
| 80 | 355.1 | 47342.64 |
| 85 | 433.6 | 57808.42 |
| 90 | 525.8 | 70100.71 |
| 95 | 633.9 | 84512.82 |
| 100 | 760.0 | 101324.7 |
| Agua K w y pK w a diferentes temperaturas (°C) | ||
|---|---|---|
| Temperatura | K w 10 —14 | pK w 2 |
| 0 | 0.112 | 14.95 |
| 5 | 0.182 | 14.74 |
| 10 | 0.288 | 14.54 |
| 15 | 0.465 | 14.33 |
| 20 | 0.671 | 14.17 |
| 25 | 0.991 | 14.00 |
| 30 | 1.432 | 13.84 |
| 35 | 2.042 | 13.69 |
| 40 | 2.851 | 13.55 |
| 45 | 3.917 | 13.41 |
| 50 | 5.297 | 13.28 |
| 55 | 7.080 | 13.15 |
| 60 | 9.311 | 13.03 |
| 75 | 19.95 | 12.70 |
| 100 | 56.23 | 12.25 |
| Capacidad calorífica específica para el agua |
|---|
| C° (H 2 O (l)) = 4184 J∙K −1 ∙kg −1 = 4,184 J∙g -1 ∙°C -1 |
| C° (H 2 O (s)) = 1864 J∙K −1 ∙kg −1 |
| C° (H 2 O (g)) = 2093 J∙K −1 ∙kg −1 |
| Temperaturas estándar de fusión y ebullición del agua y entalpías de las transiciones | ||
|---|---|---|
| Temperatura (K) | Δ H (kJ/mol) | |
| fundiendo | 273.15 | 6.088 |
| hirviendo | 373.15 | 40.656 (44.016 a 298 K) |
| Constantes crioscópicas de agua (depresión del punto de congelación) y ebullioscópicas (elevación del punto de ebullición) |
|---|
| K f = 1.86°C∙kg∙mol −1 (constante crioscópica) |
| K b = 0.49 c∙kg∙mol −1 (constante ebuloscópica) |
<figure class="ui-has-child-figcaption" id="CNX_Chem_00_EE_LiqWatAbso">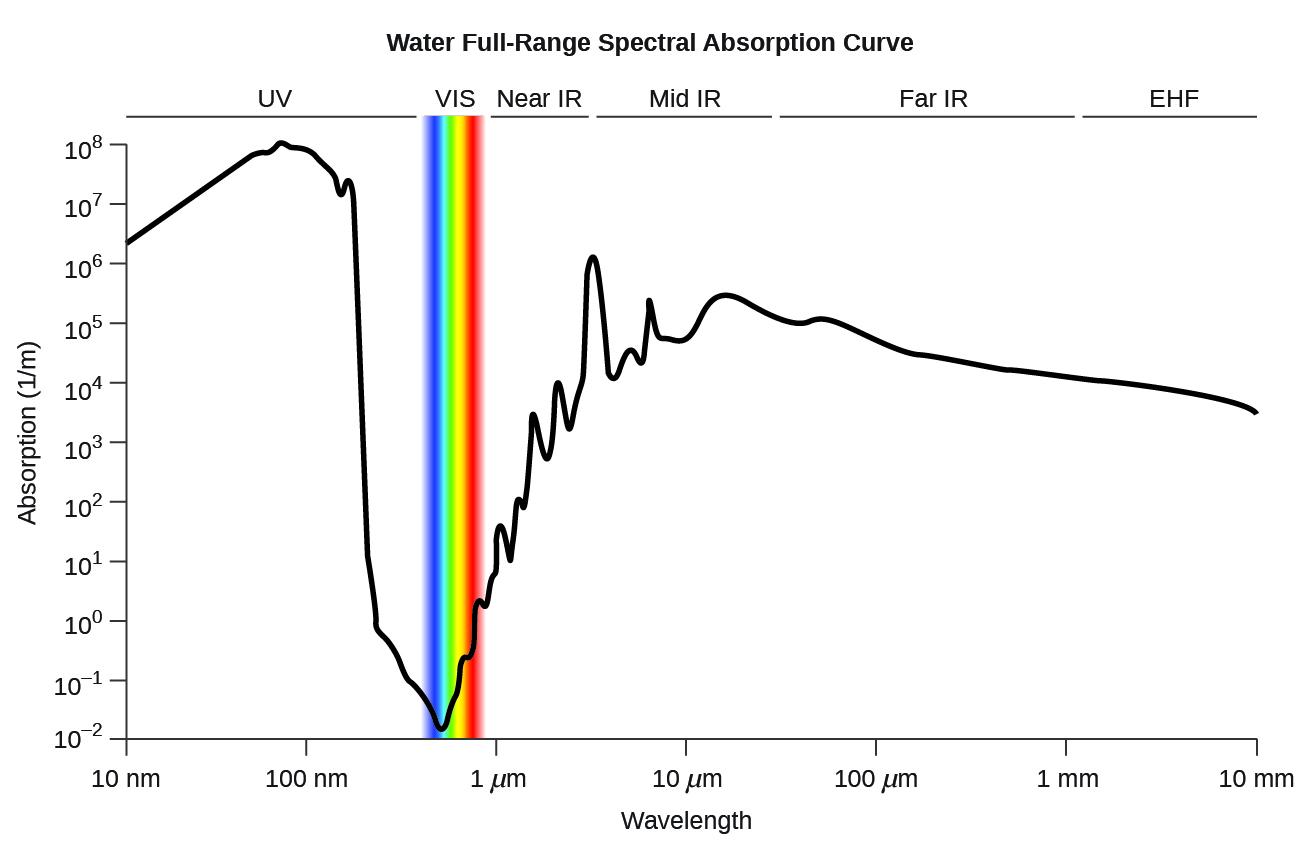 <figcaption>Curva de absorción espectral de rango completo de agua. Esta curva muestra la absorción espectral de rango completo para el agua. El eje y significa la absorción en 1/cm. Si dividimos 1 por este valor, obtendremos la longitud del camino (en cm) después de lo cual la intensidad de un haz de luz que pasa por el agua decae por un factor de la base del logaritmo natural e (e = 2.718281828). </figcaption></figure>
<figcaption>Curva de absorción espectral de rango completo de agua. Esta curva muestra la absorción espectral de rango completo para el agua. El eje y significa la absorción en 1/cm. Si dividimos 1 por este valor, obtendremos la longitud del camino (en cm) después de lo cual la intensidad de un haz de luz que pasa por el agua decae por un factor de la base del logaritmo natural e (e = 2.718281828). </figcaption></figure>





