11.9: Problems for Chapter 11
- Page ID
- 2398
Link to Solution Manual
P11.1: Propose a mechanism for the following reaction:
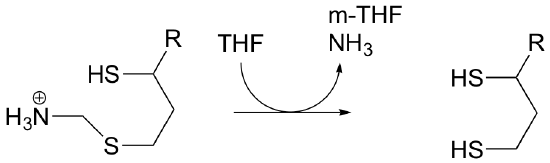
P11.2: Propose a mechanism for the following reaction:
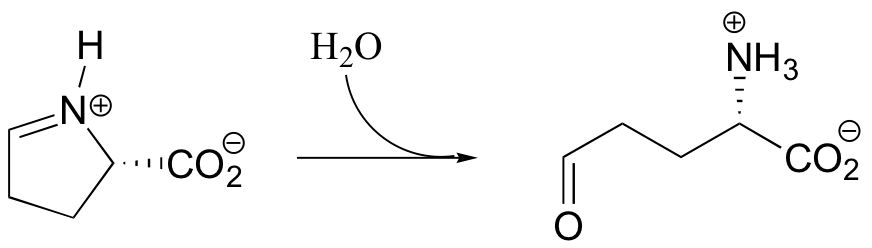
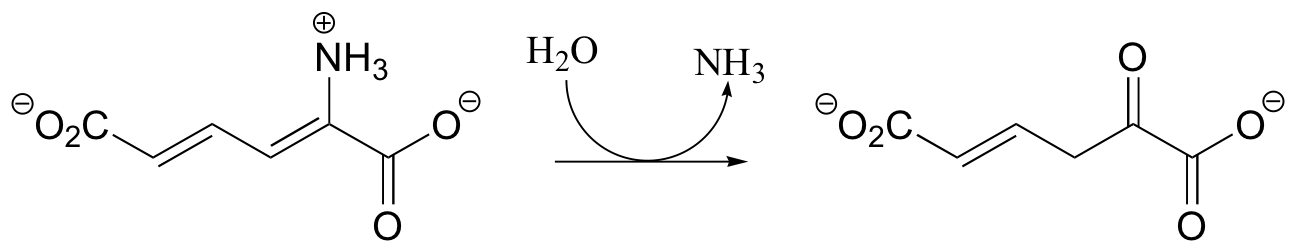
P11.3:
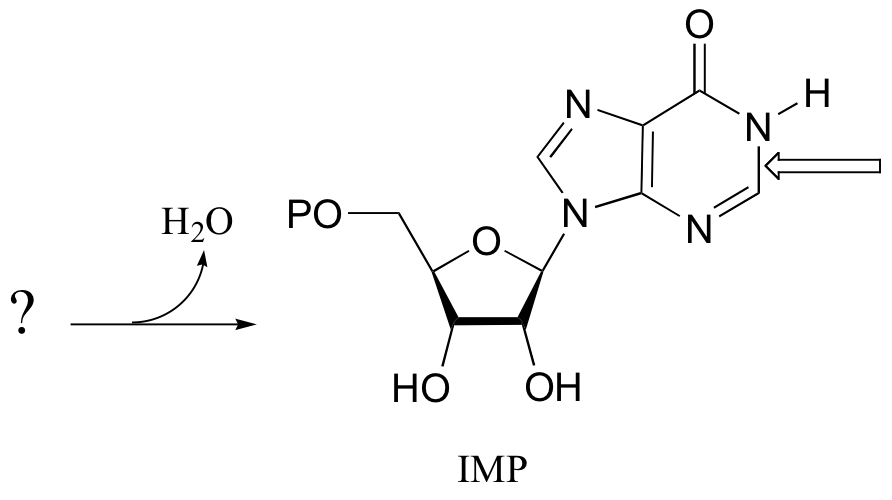
The final step in the biosynthesis of inosine monophosphate (IMP, a precursor to both AMP and GMP), is a ring-closing reaction in which a new nitrogen-carbon bond (indicated by an arrow in the structure below) is formed. Predict the starting substrate for this reaction, and propose a mechanism.
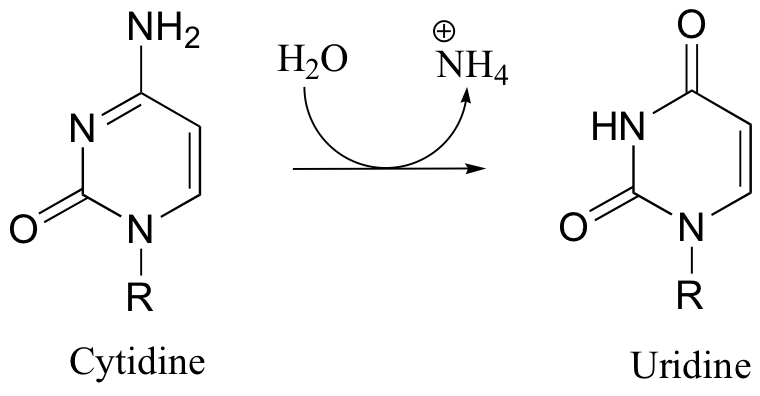
P11.4: Propose a mechanism for the conversion of cytidine to uridine:
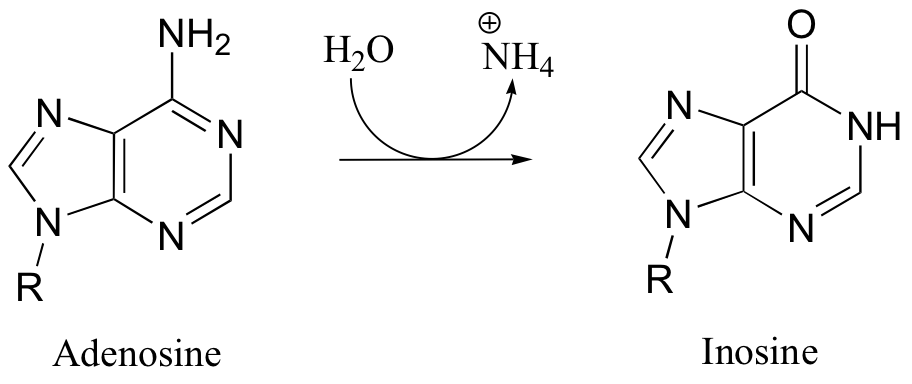
P11.5: Propose a mechanism for the first step in the degradation of adenosine:
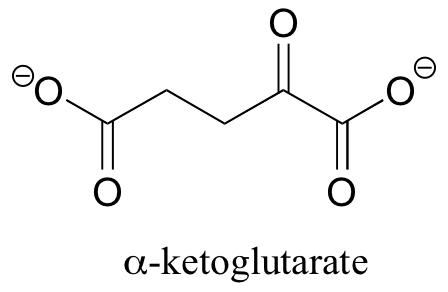
P11.6: Draw a mechanism showing the formation of an imine linkage between the side-chain amine of lysine and alpha-ketobutyrate (this is the first step in the degradation of lysine).
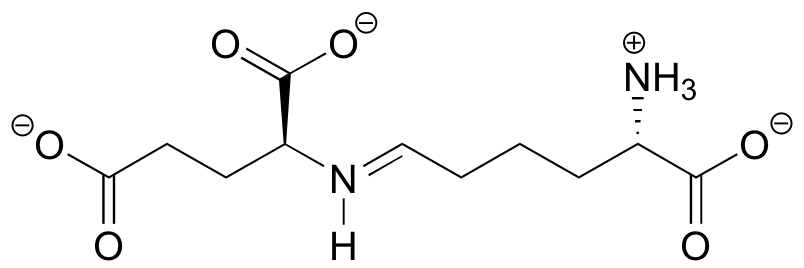
P11.7: A downstream intermediate in the lysine degradation pathway undergoes imine hydrolysis to release two amino acid products. Draw a mechanism for this hydrolysis reaction, and show the structures of the two products formed.
P11.8: Propose a mechanism for the following reaction:
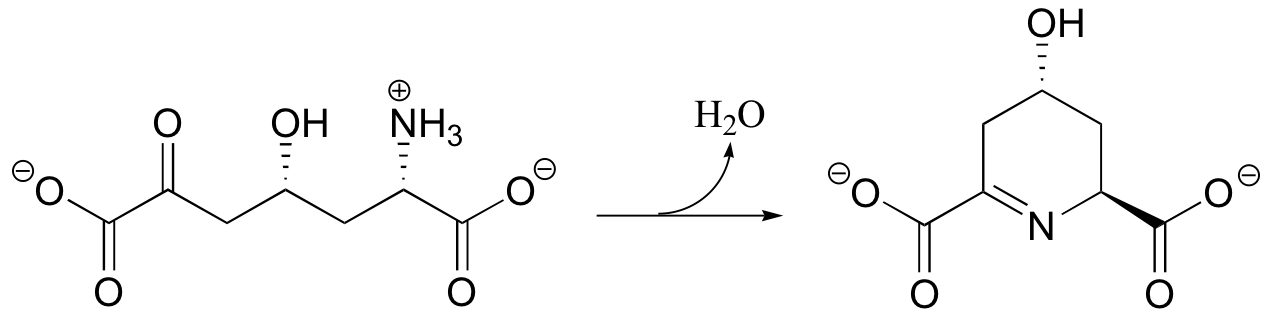
P11.9: Predict the likely product of this imine hydrolysis reaction, including the configuration of any stereocenters.
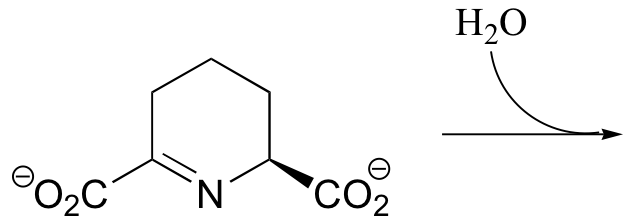
P11.10: Draw the product of the reaction indicated below, including the configuration of any stereocenters.
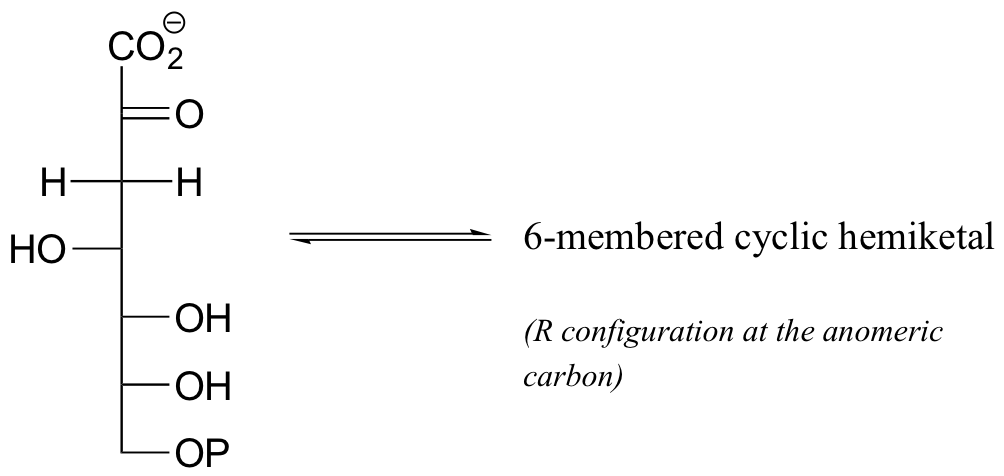
P11.11: Draw the structure (including stereochemistry) of the compound that results when the cyclic hemiketal shown below converts to a straight-chain compound with two ketone groups.
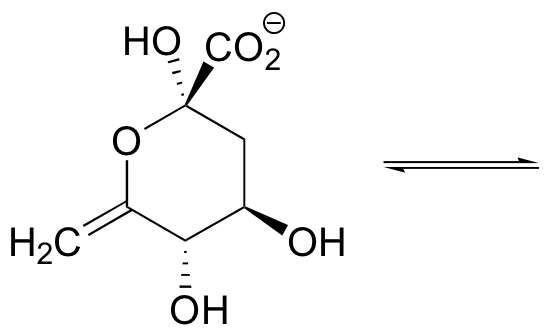
P11.12: The compound shown below undergoes a ring-opening reaction to form an enol. Draw the structure (including stereochemistry) of this product, and a mechanism for its formation.
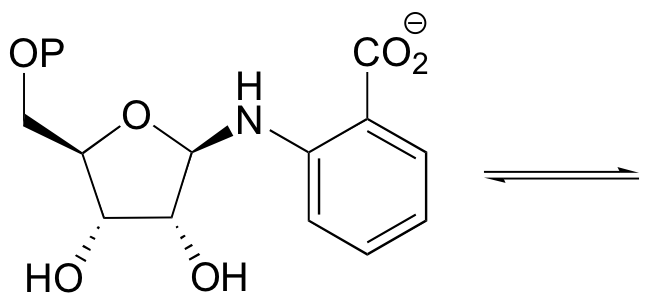
P11.13:
a) 5,10-methylene tetrahydrofolate serves as a single carbon donor coenzyme in many biosynthetic pathways, most notably in the biosynthesis of nucleotides. One way that it is formed is through the transfer of a single carbon, in the form of formaldehyde, to tetrahydrofolate (THF). Propose a mechanism for this reaction. (The entire structure of THF is shown for your information, but you should use the abbreviated structure as shown).
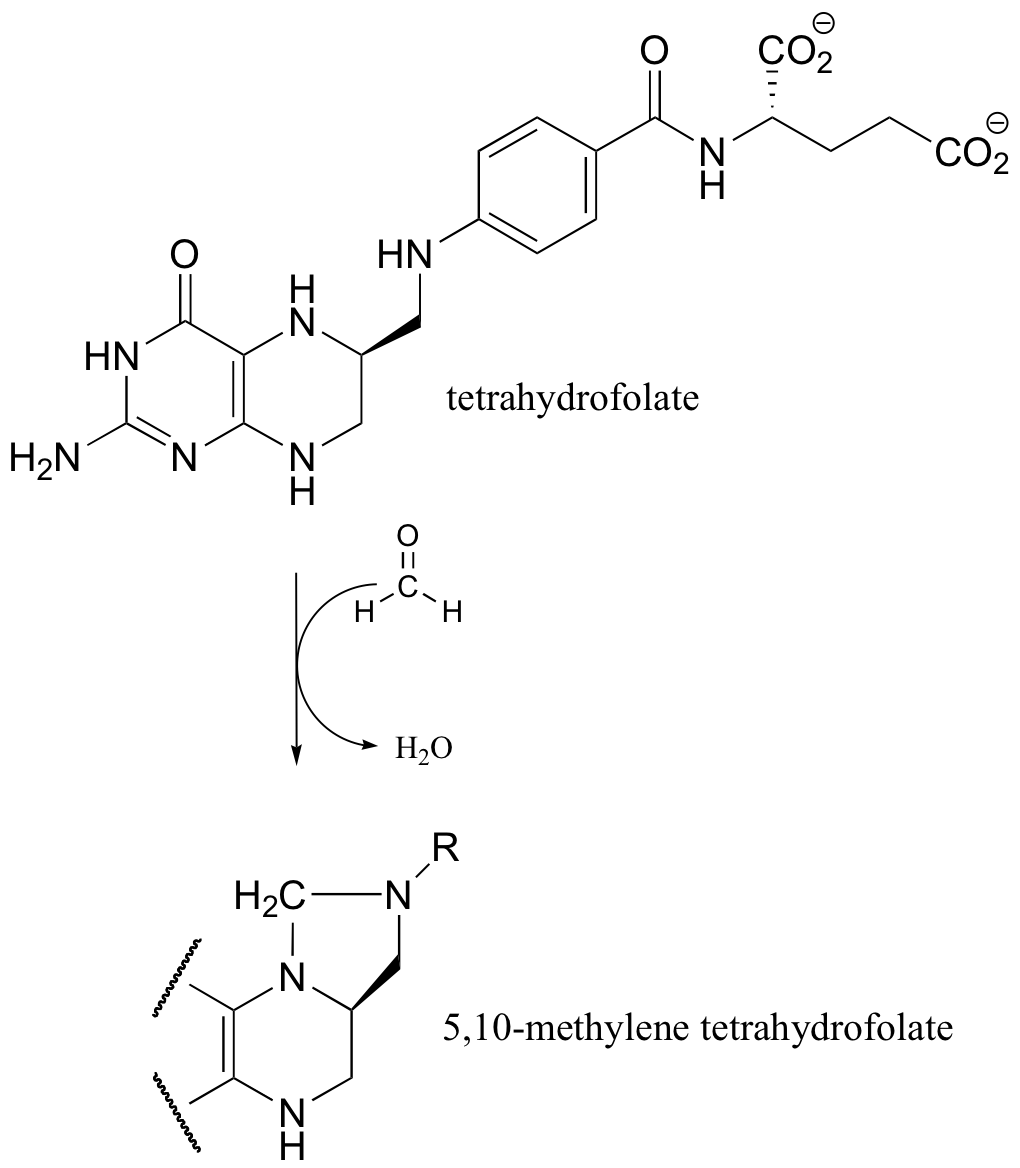
b)
Another way in which THF can act as a single electron acceptor is illustrated in the reaction below, which is a step in the histidine degradation pathway.
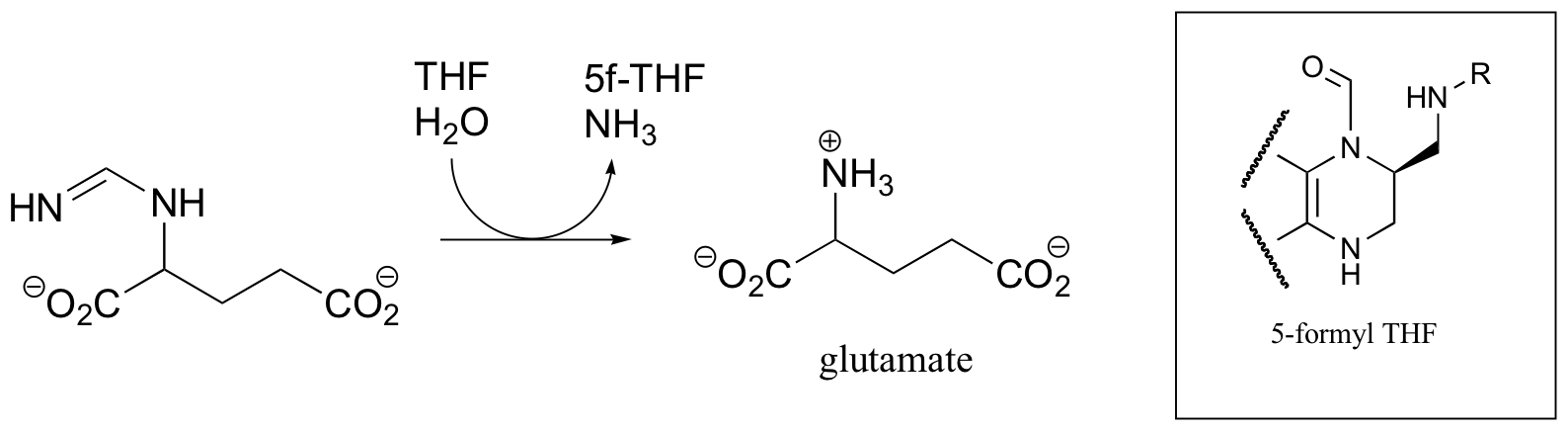
In this reaction, the single carbon is first transferred to THF as an imine, which is then hydrolized to 5-formyl-THF. Draw a complete mechanism.
P11.14: Propose a mechanism for the following reaction:
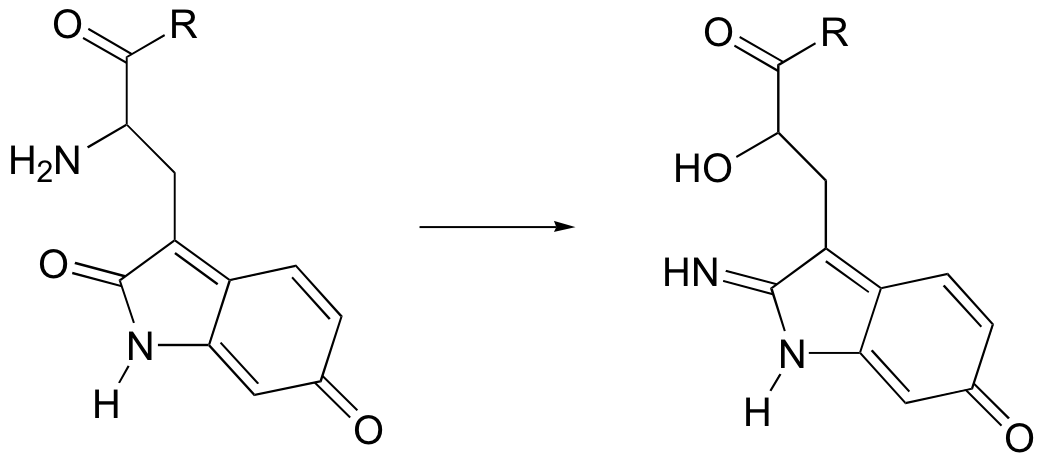
P11.15: You probably know that ascorbic acid (vitamin C) acts as an antioxidant in the body. When vitamin C does its job, it ends up being oxidized to dehydroascobate, which is usually drawn as shown below in the so-called tricarbonyl form.
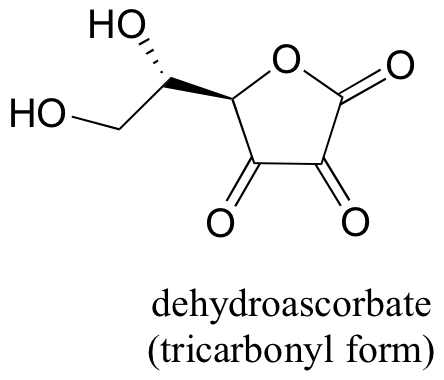
It is thought, however, that the most important form of dehydroascobate in a physiological context is a hydrated, hemiketal form (see the "Insights: Chemical Footnote" section on p. 36 of Chemical and Engineering News, Aug. 25, 2008). Show the structure of this form of dehydroascorbic acid, and a mechanism for its (enzyme-free) formation from the tricarbonyl form.
Challenge problems
C11.1: Propose a mechanism for the following transformation:
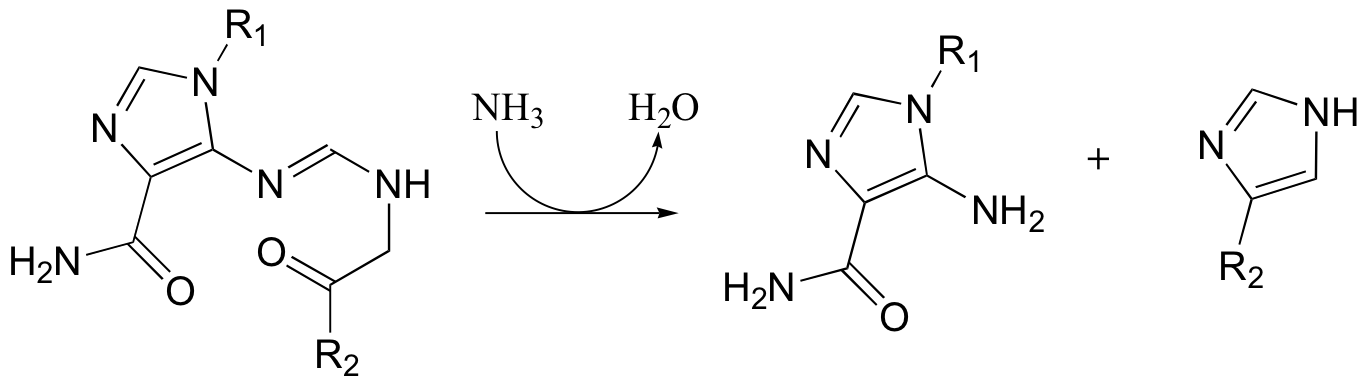
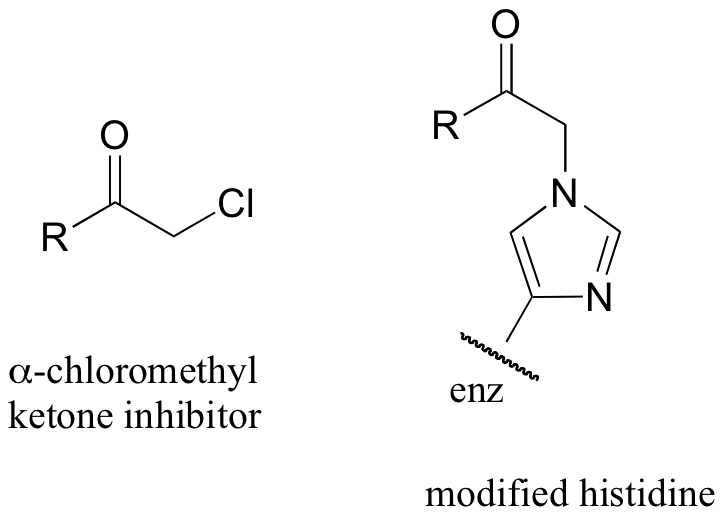
C11.2: alpha-chloromethyl ketones (structure below) are effective irreversible inhibitors of proteolytic (peptide-bond breaking) enzymes such as chymotrypsin. In these enzymes, a nucleophilic serine plays a key role in the reaction. The mechanism for inactivation of alpha-chymotrypsin is thought to involve, as a first step, nucleophilic attack by the active site serine on the carbonyl of the inhibitor. However, when the inactivated enzyme is analyzed, an active site histidine rather than the serine, is found to be covalently modified by the inhibitor. The structure of the modified histidine is shown below. The mechanism of inactivation is thought to involve an epoxide intermediate - with this in mind, propose a reasonable mechanism of inactivation.
C11.3: An enzyme in E. coli bacteria catalyzes the hydrolysis of alpha-glucose-GDP to glucose.
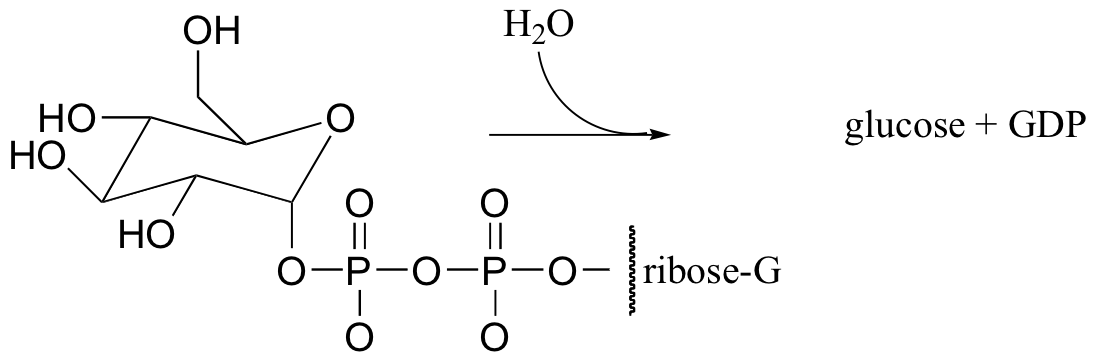
1H-NMR analysis of the reaction in progress showed the initial appearance of a doublet at 4.64 ppm with J = 7.9 Hz (the spectrum contained other signals as well, of course). After 20 minutes (at which point the hydrolysis reaction has been complete for some time), another doublet began to appear slightly downfield, this one with J = 4.0 Hz. Over time, the strength of the downfield signal gradually increased and that of the upfield signal gradually decreased, until they stabilized at constant levels.
Draw a mechanism for the enzymatic hydrolysis reaction, and correlate your mechanism to the NMR data (including the appearance of the second doublet).
C11.4: Arginine deaminase, an enzyme in the arginine degradation pathway, catalyzes the transformation of (L)-arginine to (L)-citrulline via a covalent substrate-cysteine intermediate.
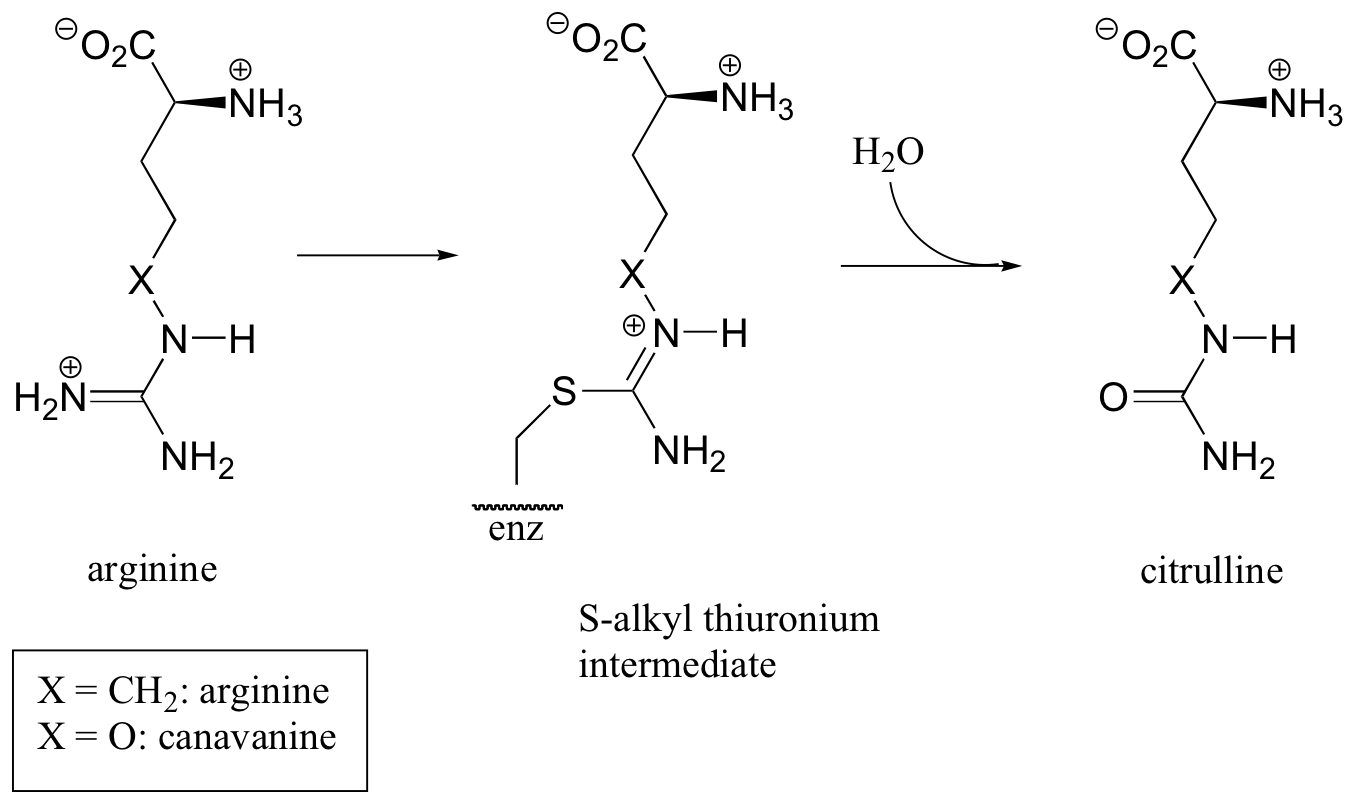
This enzyme is the target for the development of drugs for cancer and immunological diseases such as arthritis. However, rather than completely and permanently shutting down the enzyme (eg. with an irreversible inhibitor), researchers are looking for a way to temporarily 'turn down' the activity of the enzyme. One strategy that has recently been reported involves the use of an oxygen-containing arginine analog, called canavanine, which reacts in the same way as arginine except that the second (hydrolysis) step is very slow. While the enzyme is covalently attached to the inhibitor (in the S-alkyl thiuronium stage), it is inactivated.
a) Show a mechanism for the reaction catalyzed by arginine deaminase.
b) Explain how the electronic effect of the oxygen substituent would slow down the hydrolysis step of the reaction, and why the rate of the hydrolysis step is more affected by the oxygen substitution than the S-alkylthiuronium-forming step.
C11.5:The final step in the degradation pathway for the amino acid glycine (also known as the 'glycine cleavage system') is shown below. Propose a likely mechanism, given what you know about the tetrahydrofolate coenzyme from section 11.6D. Evidence suggests (see reference in solutions manual) that CH2NH2+ is an intermediate.