15.11: Problems for Chapter 15
- Page ID
- 2437
Link to Solutions Manual
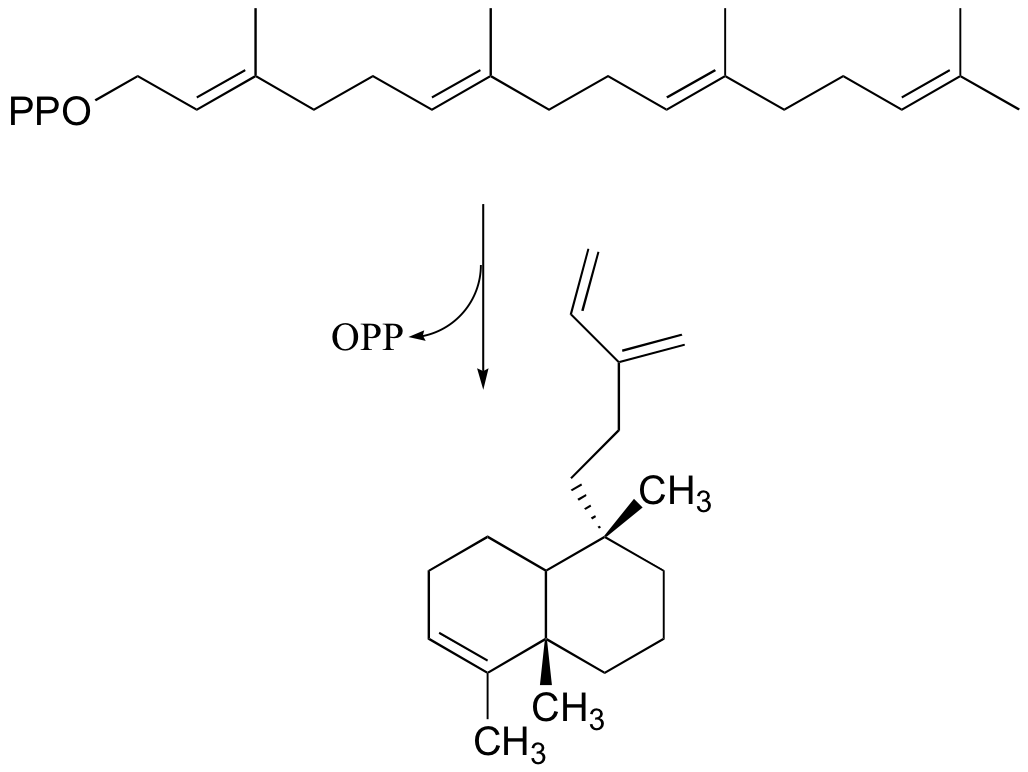
P15.1: Provide a mechanism for the following SAM-dependent reaction:
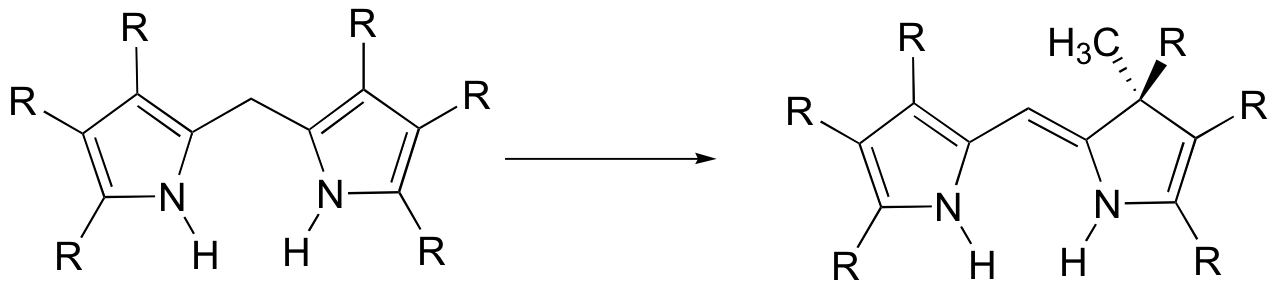
P15.2: Provide a mechanism for the following reaction:
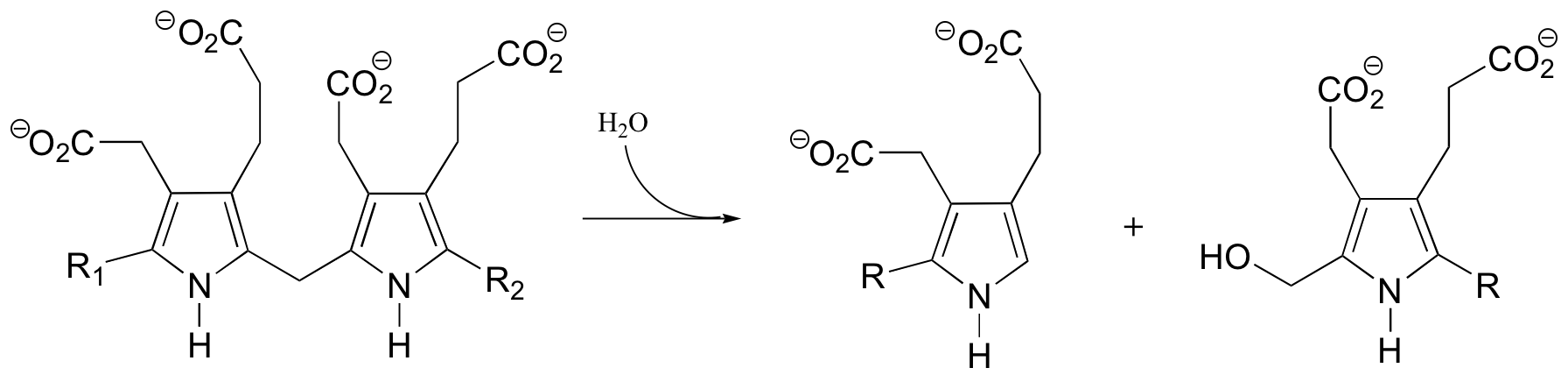
P15.3: Consider the two possible results of the isotopic labeling experiment shown below for the reaction catalyzed by 3-deoxy-D-manno-octulosonate-8-phosphate synthase:
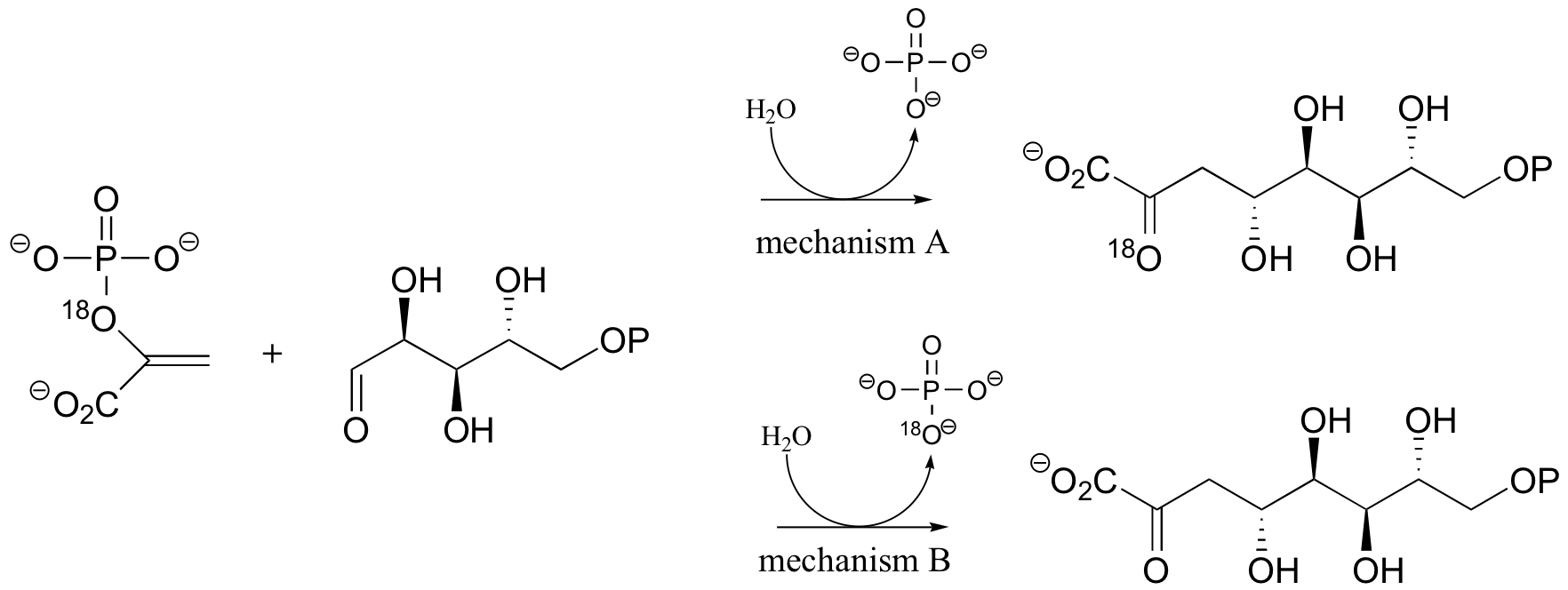
a) Propose a mechanism that is consistent with result A.
b) Propose a mechanism that is consistent with result B.
c) Literature research question: Which result was actually observed?
P15.4: Consider the following isomerization:
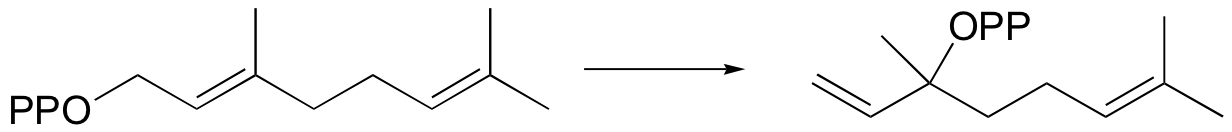
a) Suggest a likely mechanism involving a carbocation intermediate.
b) Suggest an isotopic labeling experiment that might rule out a concerted isomerization reaction.
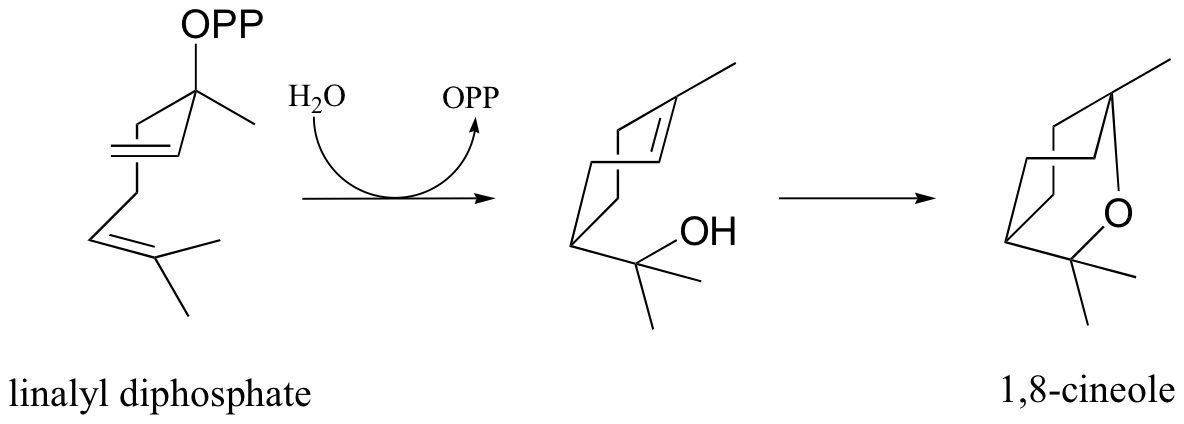
P15.5: Provide a mechanism for the following reaction (notice that the starting compound is linalyl diphosphate, the product from problem above, drawn in a different perspective)
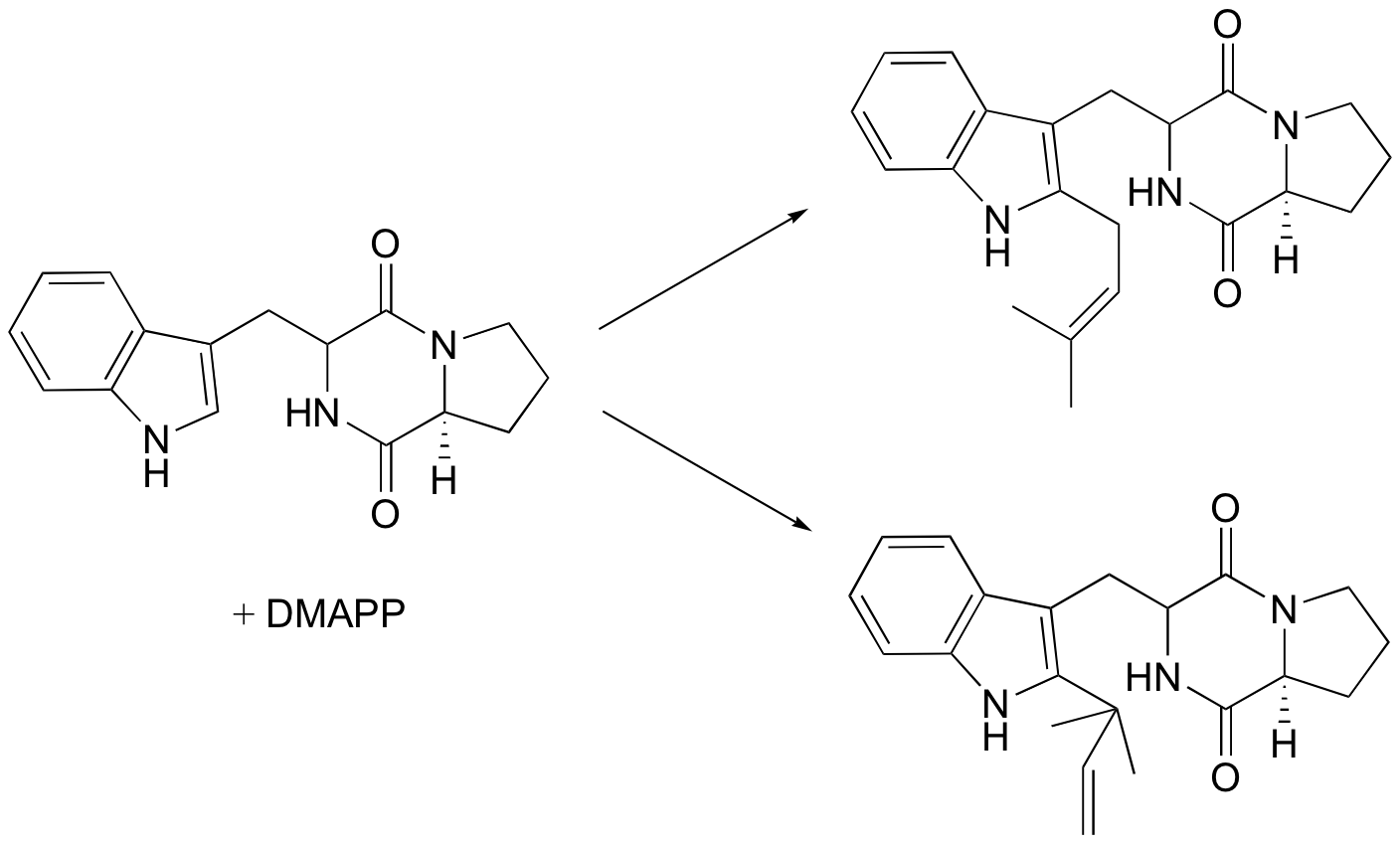
P15.6: Draw mechanisms for the following reactions, both of which are part of an alkaloid synthesis pathway in fungi.
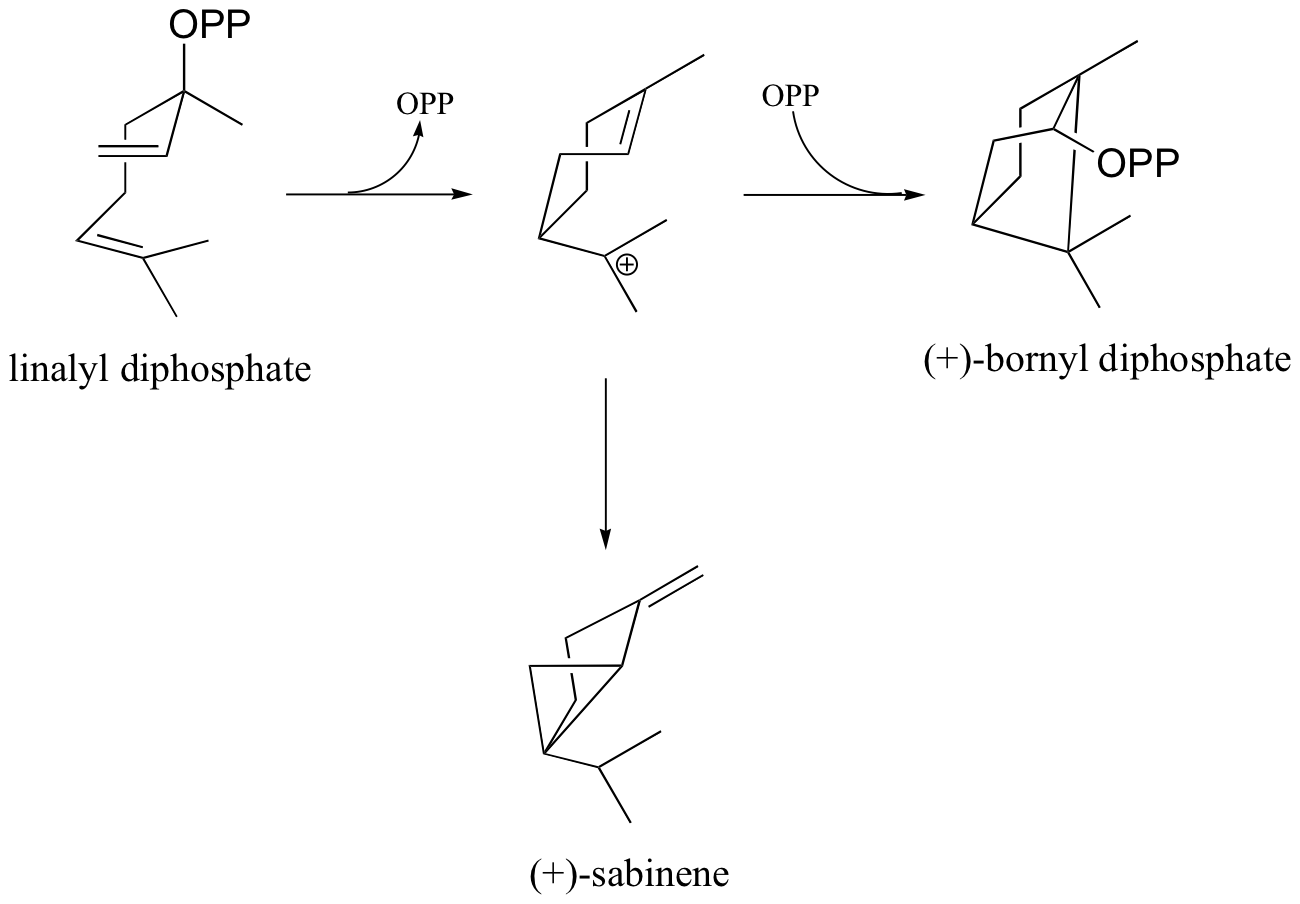
P15.7: Provide mechanisms for the conversion of linalyl diphosphate to
a) (+)-bornyl diphosphate
b) (+)-sabinene.
c) Is the second step in the (+)-bornyl diphosphate pathway (addition of phosphate) a Markovnikov or anti-Markovnikov addition? Explain.
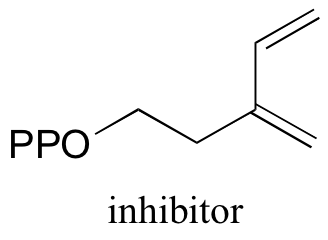
P15.8: A diene molecule was found to be an irreversible inhibitor of isopentenyl diphosphate isomerase. The enzyme is known to have an active site cysteine that acts as a catalytic acid/base in the reversible isomerase reaction. Propose a likely mechanism for irreversible inhibition.
P15.9:Electrophilic addition of water to alkynes generally results in the formation of a ketone (or possibly an aldehyde, depending on the starting alkyne). Predict the product of the following reaction, and show a mechanism for its formation.
![]()
P15.10: Methyl vinyl ketone (structure below) was treated with HBr, and the product isolated and analyzed by 1H NMR.
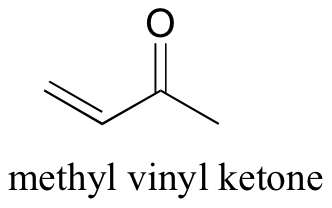
The following data were obtained:
2.2 ppm (3H, s)
3.0 ppm (2H, t)
3.5 ppm (2H, t)
Give the structure of the product, and explain the observed regiochemistry.
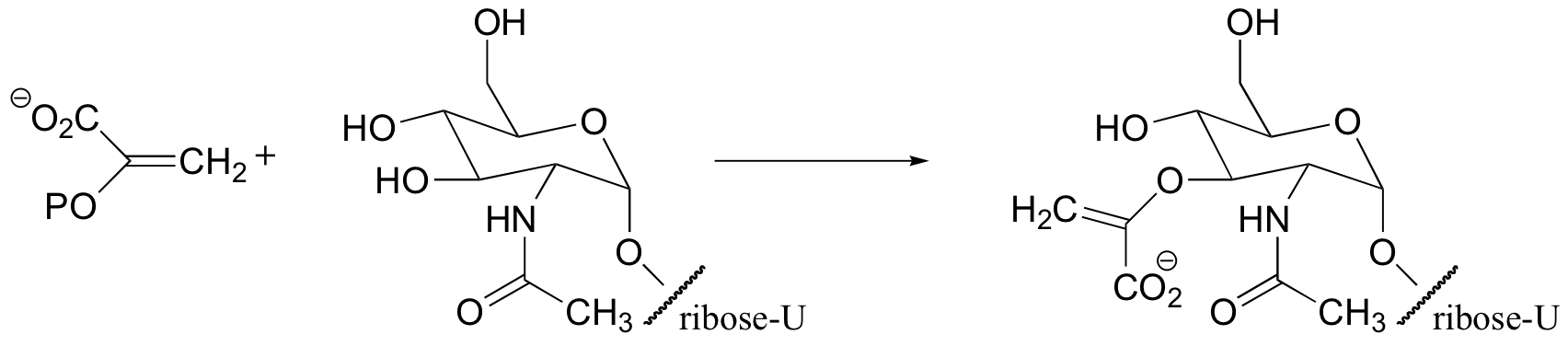
P15.11: Suggest a likely mechanism for this reaction, which is a key step in the synthesis of bacterial cell walls:
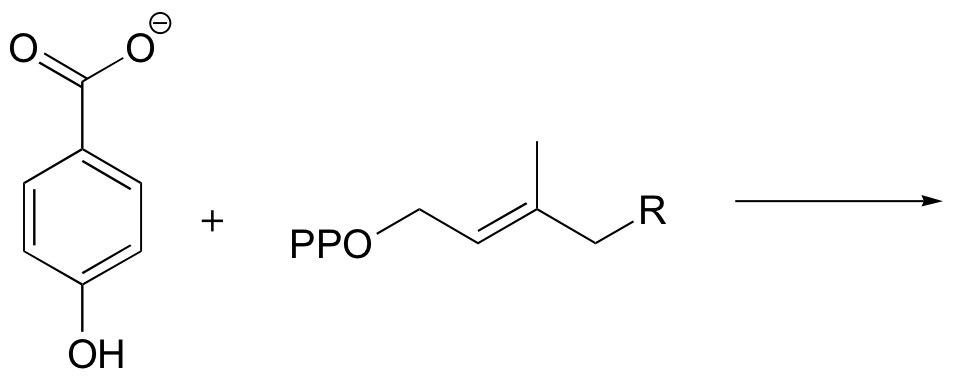
P15.12: In the following reaction, which is part of the biosynthetic pathway for vitamin K and other 'quinone' compounds, an aromatic ring carbon is alkylated. Predict the structure of the product, and use a mechanistic argument to explain your prediction.
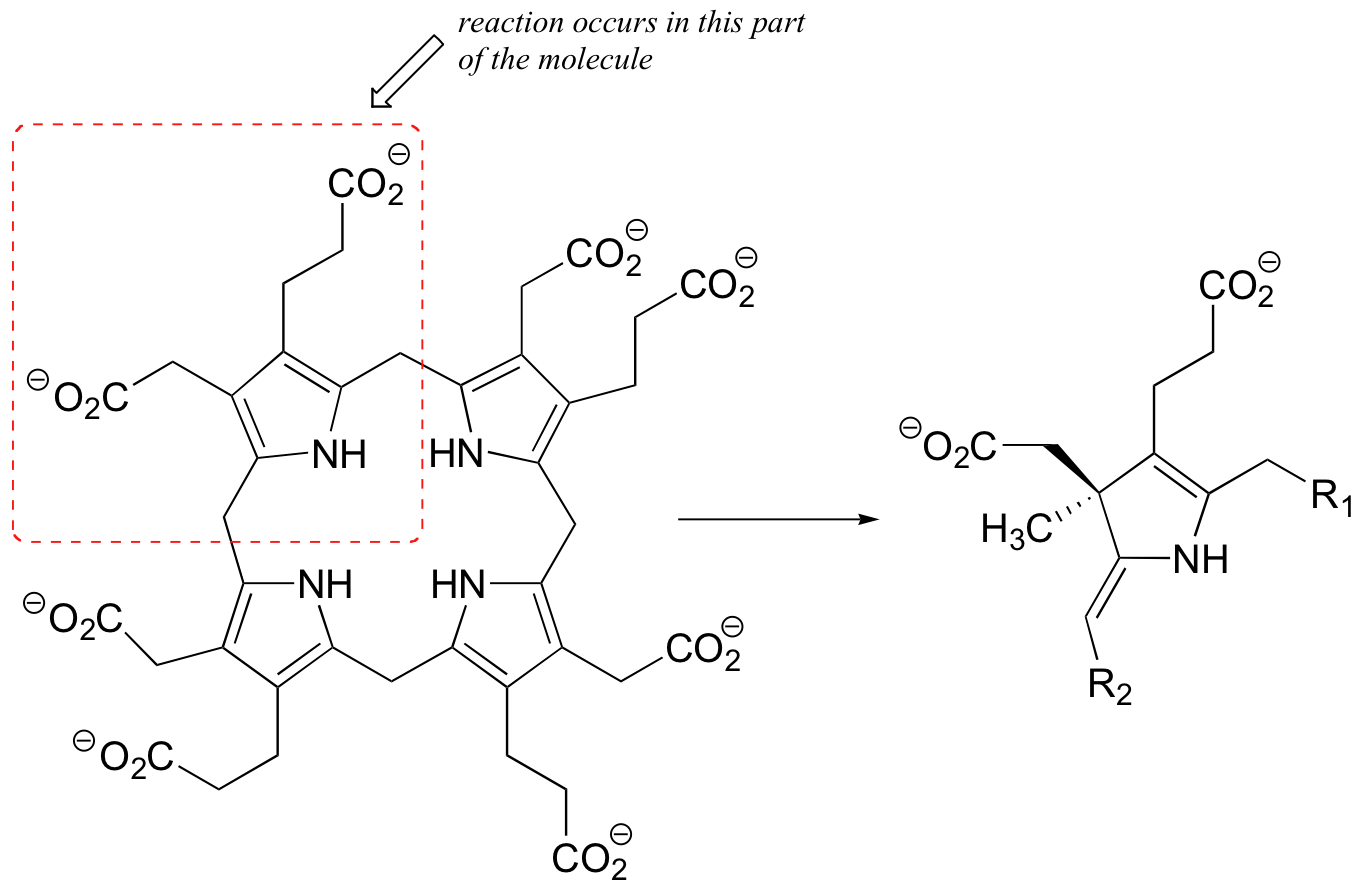
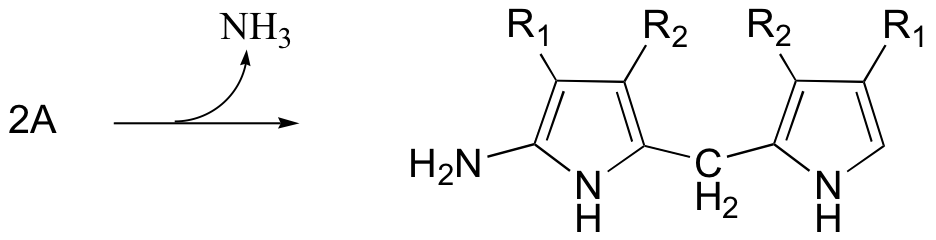
P15.13: Propose a mechanism for the following reaction from vitamin B12 biosynthesis. An enzyme cofactor (not shown) is required for this reaction - which one? Account for the regioselectivity of the reaction (in other words, show how the relevant carbocation intermediate is stabilized). Use R1 and R2 as in the figure below to abbreviate.
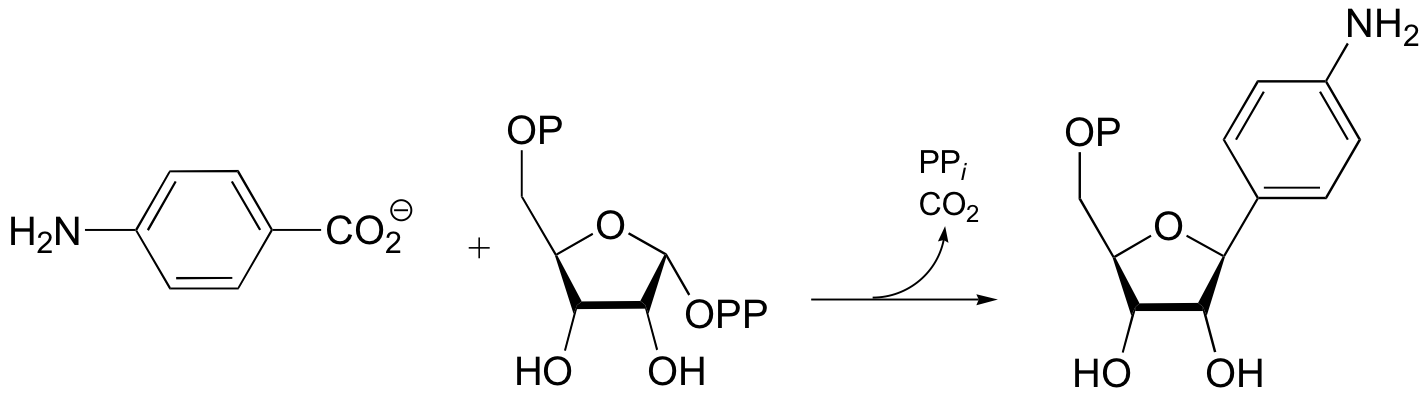
P15.14: Suggest a mechanism for the following aromatic alkylation. This reaction is part of the pathway by which many microbes synthesize methanopterin, a derivative of the vitamin folic acid.
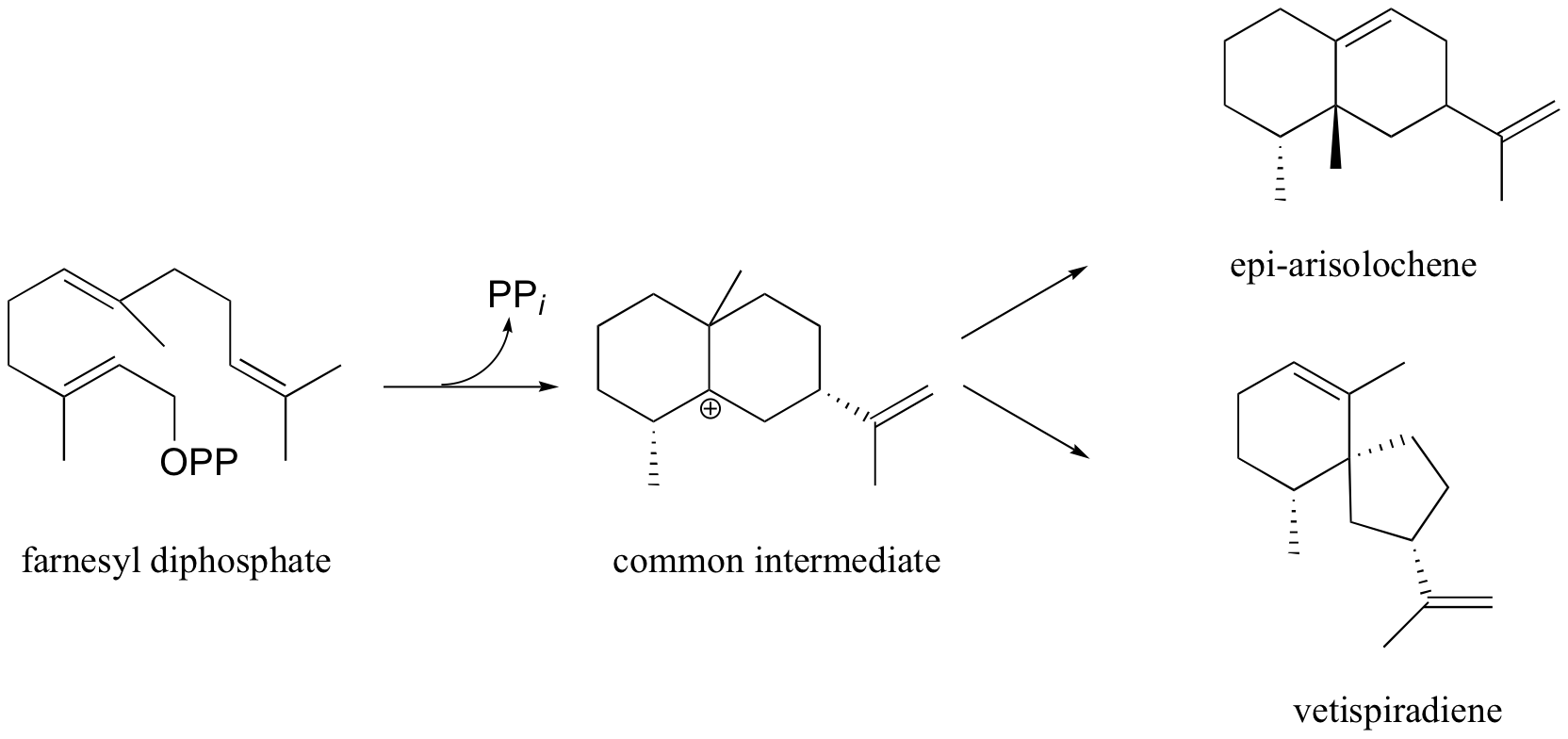
P15.15: Suggest mechanisms for the cyclization of farnesyl diphosphate to:
a) epi-aristolochene
b) vetispiradiene
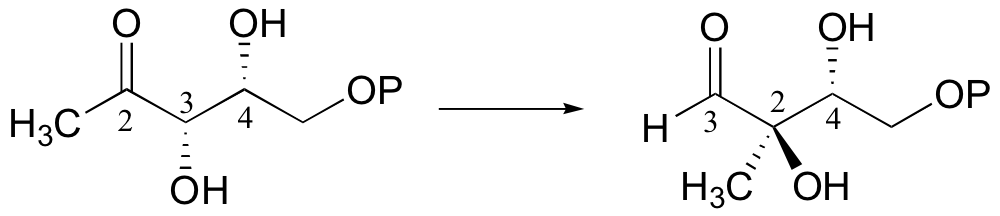
P15.16: Suggest a likely mechanism for the following enzymatic reaction, which is part of the early stage of isoprenoid biosynthesis in bacteria and plants. A carbon numbering scheme is included to help you to follow the rearrangement.
P15.17: The pyrophosphate leaving group in the cyclopropane-forming part of the squalene synthase reaction is protonated by an active site tyrosine that is positioned close to C1 of one of the FPP molecules. Speculate as to why evolution may have specifically favored the selection of tyrosine as the acid group in this step.
P15.18: Draw mechanisms for the (nonenzymatic) reactions below.
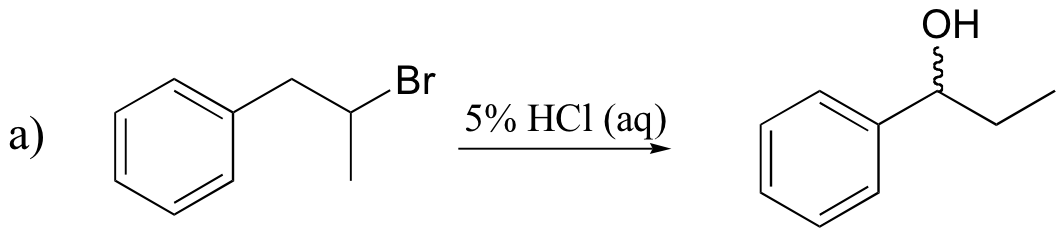
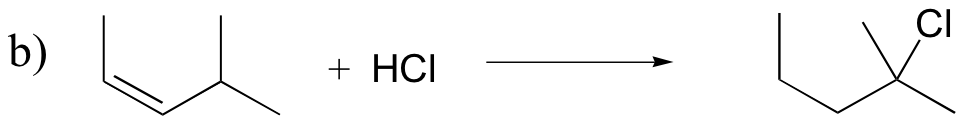
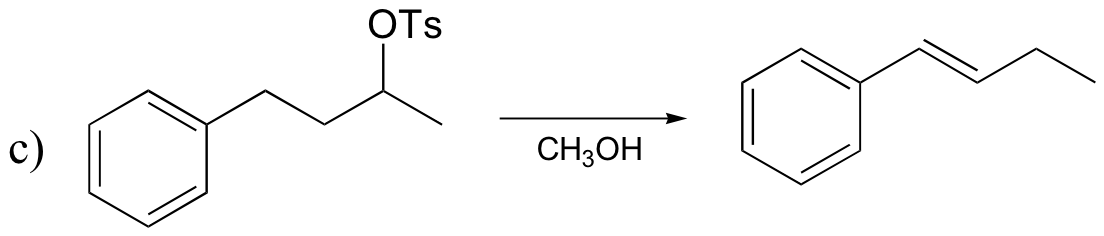
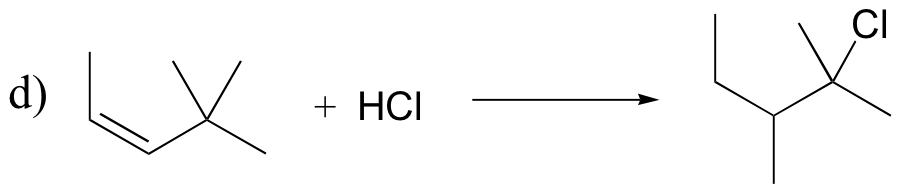
P15.19: Draw the structure of the main organic products of the following reaction sequences. When appropriate, indicate the formation of multiple stereoisomers.
a)
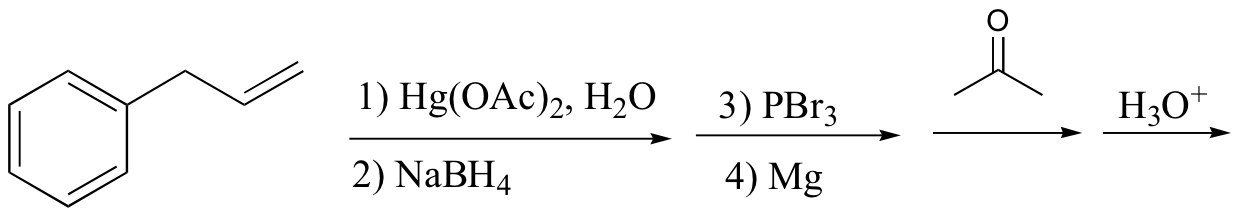
b)
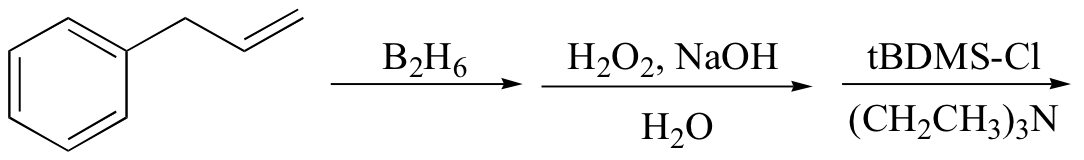
c)
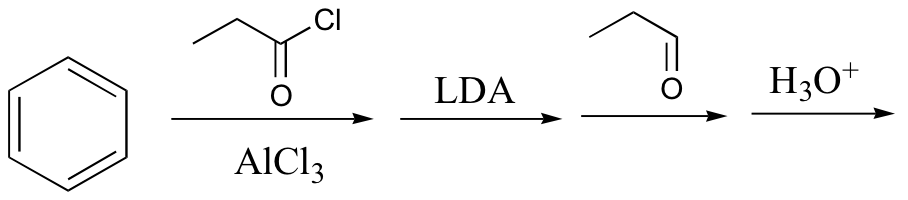
d)
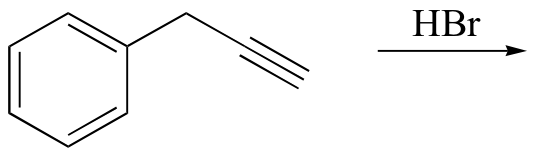
e)
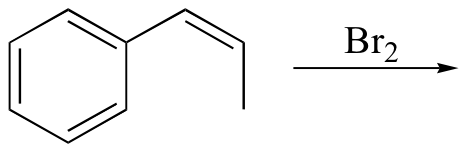
f)
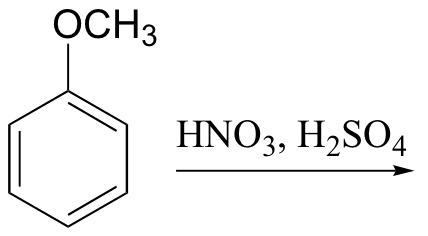
g)
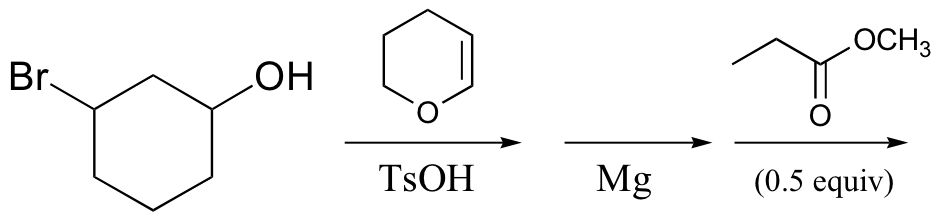
h)
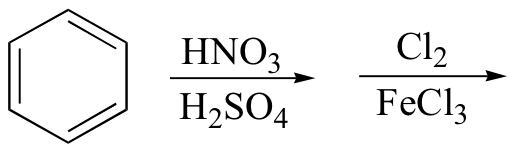
i)
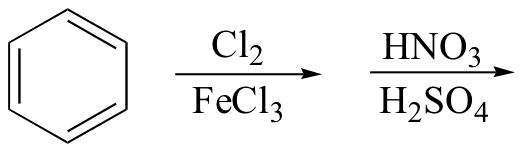
j)
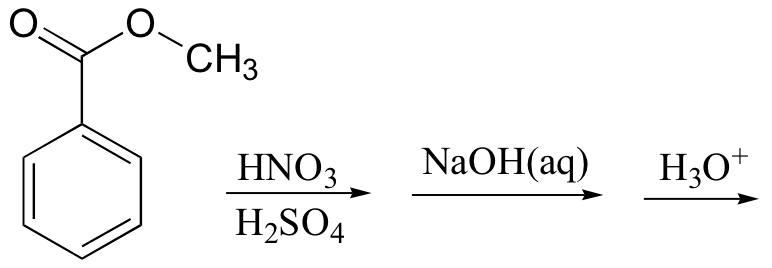
k)
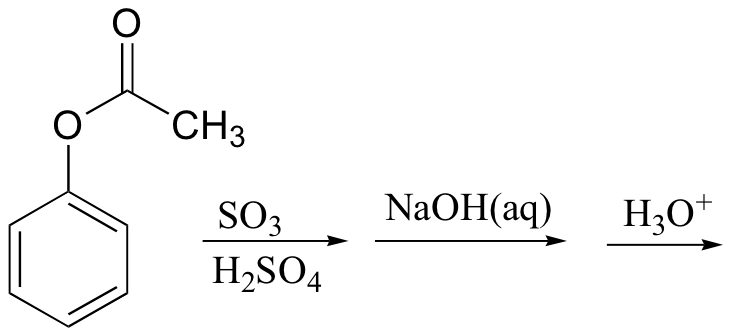
15.20: Propose routes for the following multistep syntheses. Use any synthetic reagents that are appropriate, along with the organic starting materials given.
a)
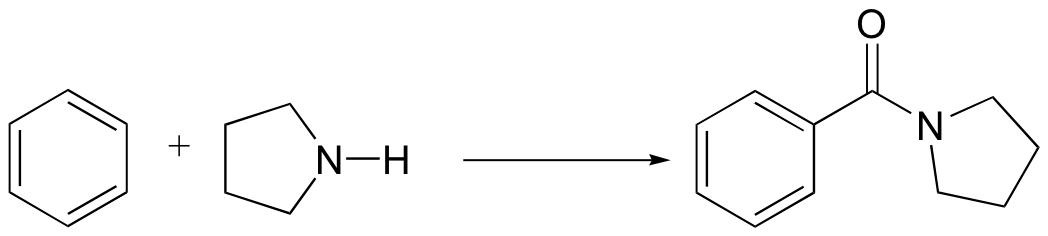
b)
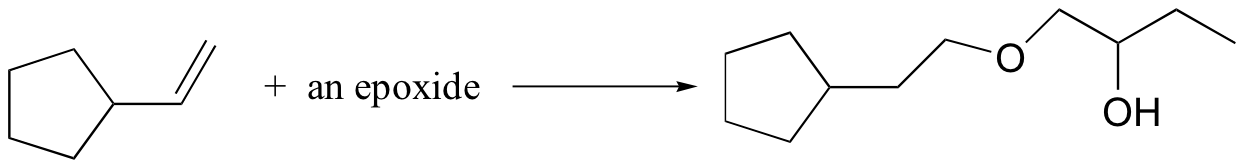
c)
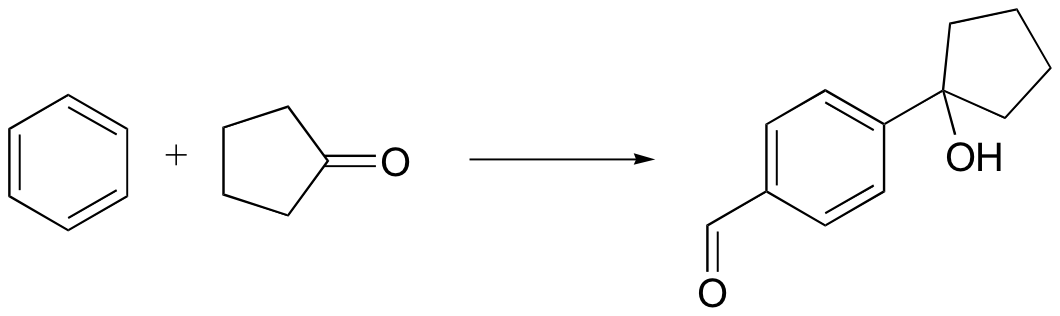
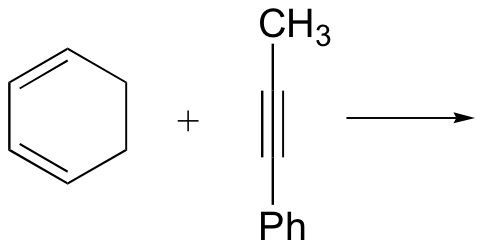
P15.21: Predict the products of the following Diels-Alder reactions:
a)
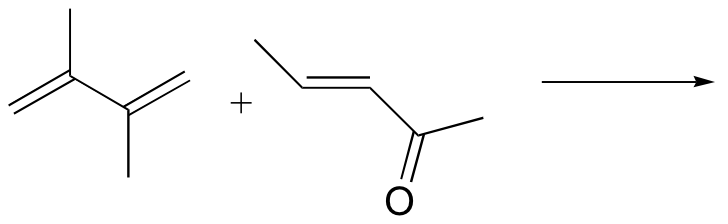
b)
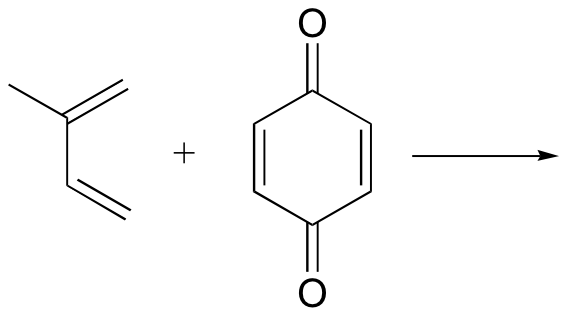
c)
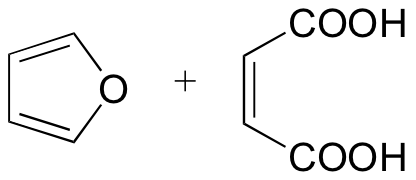
d)
P15.22: Draw structures of the diene and dienophile molecules that could be used to obtain the following Diels-Alder products:
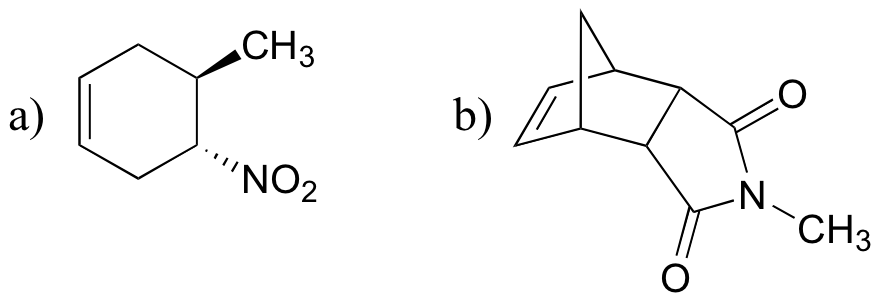
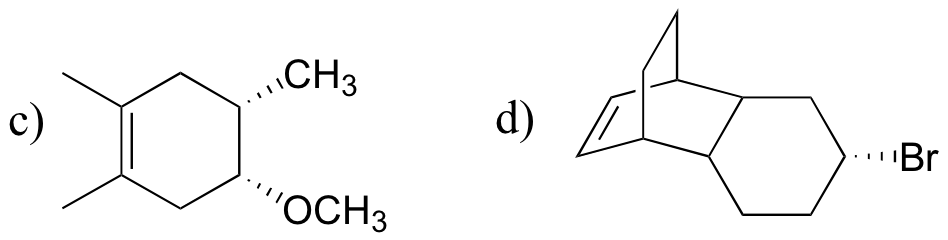
P15.23: Predict the starting compound or product of the following Cope/Claisen rearrangements.
a)
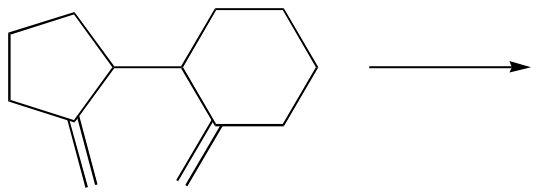
b)
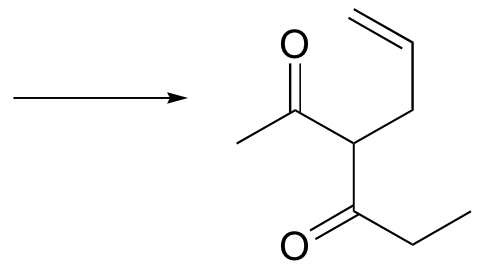
c)
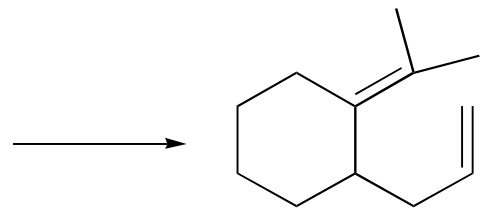
d) (the product is a phenol, and requires an additional chemical step after the rearrangement)
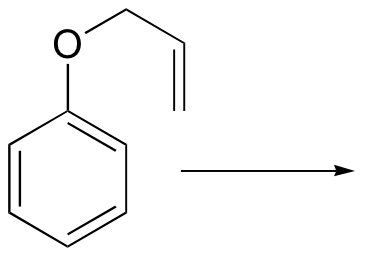
Challenge problems
C15.1: Methyl methacrylate (structure below) was treated with HBr, and the product isolated and analyzed by 1H NMR. The following data were obtained:
δ integration splitting
1.3 3H d
2.3 1H sextet
3.5 1H dd
3.6 1H dd
3.7 3H s
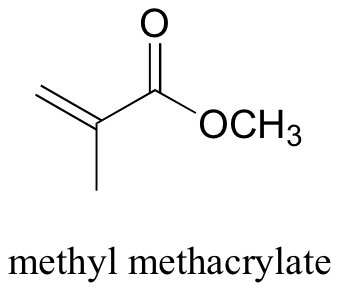
Give the structure of the product, explain the observed regiochemistry, and account for the ‘doublet of doublet’ splitting patterns in the NMR spectrum.
C15.2: In 1998, an enzyme was discovered which catalyzes a type of concerted electrophilic cyclization reaction known as a Diels-Alder reaction. This is a very well known reaction in the organic laboratory, but had never before been observed in a biological context. Propose a mechanism for this reaction (your mechanism should be concerted - ie. all bond-breaking and bond-forming events take place simultaneously).
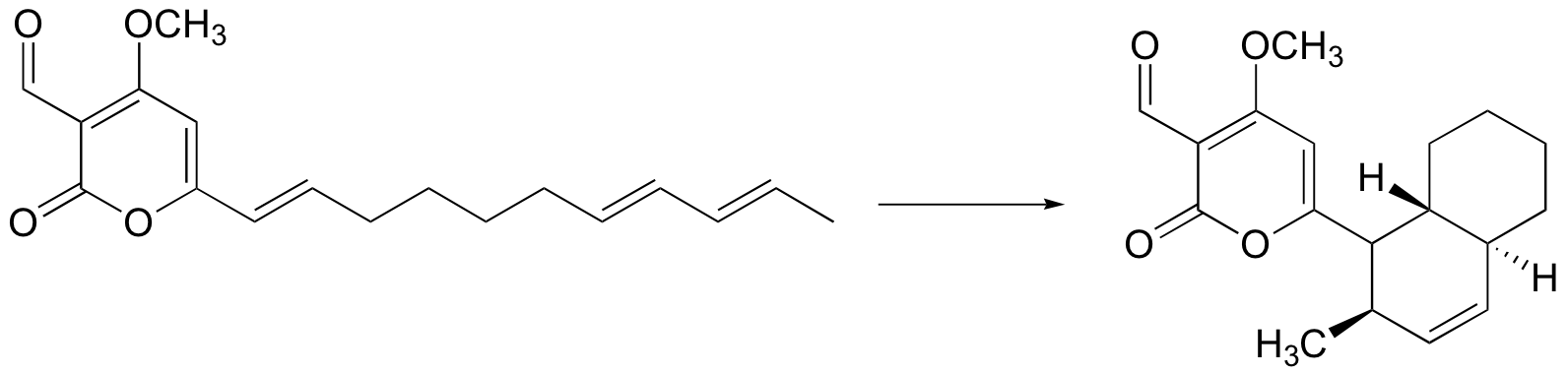
C15.3: In 2003, another enzymatic reaction was discovered which appeared to catalyze a decarboxylation followed by a Diels Alder reaction.
a) Show this proposed mechanism:
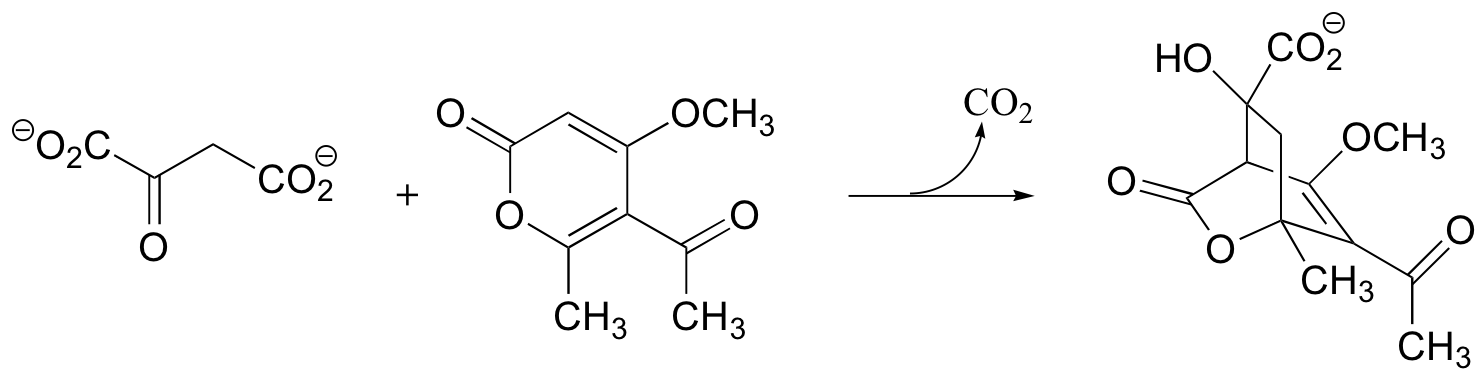
b) Later studies suggested that the cyclization reaction proceeded via a Michael addition rather than a Diels-Alder mechanism. Show this alternative mechanism (the decarboxylation is the same as in part (a), so you needn't show this). (J. Am. Chem. Soc. 2005, 127, 3577.)
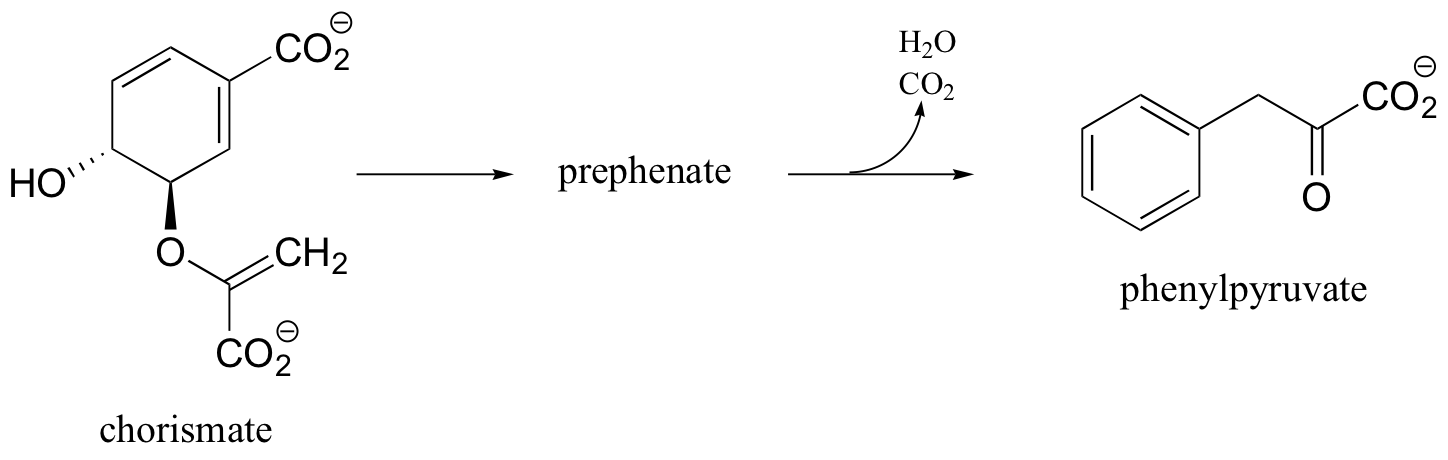
C15.4: The conversion of chorismate to phenylpyruvate is a key transformation in the biosynthesis of phenylalanine. The first step is a concerted electrophilic rearrangement to form prephenate (this step involves a six-membered transition state). Deduce the structure of prephenate, and provide a complete mechanism for the transformation.
C15.5: In the following reaction, a two molecules of the same compound condense. Show the structure of the starting molecule A, and suggest a mechanism for formation of the product.
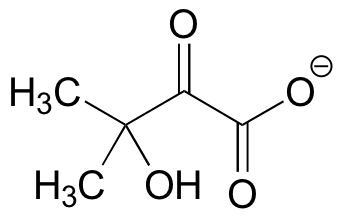
C15.6: The biosynthesis of valine begins with the condensation of two molecules of pyruvate (with the assistance of a certain coenzyme), followed by an acyloin rearrangement to form the intermediate shown below. Suggest a mechanism for this transformation, and include the role played by the coenzyme.
C15.7: Provide a mechanism for the following reaction: