10.7: Plásticos y Medio Ambiente
- Page ID
- 72095
- Conocer los problemas asociados a los plásticos.
- Identificar el tipo de polímero asociado a cada número de reciclaje.
- Conoce los diferentes procesos de reciclaje de plásticos.
Problemas con los Plásticos
Debido a su bajo costo, facilidad de fabricación, versatilidad e impermeabilidad al agua, los plásticos se utilizan en multitud de productos de diferente escala, incluyendo clips para papel y naves espaciales. Han prevalecido sobre los materiales tradicionales, como la madera, la piedra, el cuerno y el hueso, el cuero, el metal, el vidrio y la cerámica, en algunos productos previamente dejados a los materiales naturales. Sin embargo, existen numerosos problemas encontrados con el uso de plástico.
Liberación de moléculas pequeñas
Muchos tipos de polímeros contienen moléculas pequeñas, ya sea monómeros sin reaccionar o sustancias específicamente agregadas (plastificantes, absorbentes de UV, retardantes de llama, etc.) para modificar sus propiedades. Muchas de estas moléculas más pequeñas son capaces de difundirse a través del material y ser liberadas en cualquier líquido o aire en contacto con el plástico, y eventualmente en el medio acuático. Aquellos que se utilizan para materiales de construcción (en casas móviles, por ejemplo) pueden acumularse en ambientes cerrados y contribuir a la contaminación del aire interior.
Monómero residual
La formación de cadenas poliméricas largas es un proceso complicado y algo aleatorio que nunca es perfectamente estequiométrico. Por lo tanto, no es raro que algún monómero sin reaccionar permanezca en el producto terminado. Algunos de estos monómeros, como el formaldehído, el estireno (de poliestireno, incluidos los recipientes de comida para llevar de espuma de poliestireno), el cloruro de vinilo y el bisfenol-A (a partir de policarbonatos) son carcinógenos conocidos. Si bien hay poca evidencia de que las pequeñas cantidades que se difunden en el aire o se filtran hacia los fluidos representan un riesgo cuantificable para la salud, la gente es comprensiblemente reacia a tolerar estas exposiciones, y la política pública poco a poco comienza a regularlas.
El ácido perfluorooctanoico (PFOA), el monómero del que se elabora el teflón, ha sido objeto de una demanda de 2004 contra una fábrica de DuPont que contaminó las aguas subterráneas. Se han detectado pequeñas cantidades de PFOA en las emisiones gaseosas de productos de fluorocarbono calientes.
Productos de descomposición
Los polímeros más utilizados no son fácilmente biodegradables, particularmente bajo las condiciones anaeróbicas de la mayoría de los vertederos. Y lo que sí se produzca la descomposición se combinará con el agua de lluvia para formar lixiviados que puedan contaminar arroyos cercanos y suministros de agua subterránea. La fotodescomposición parcial, iniciada por la exposición a la luz solar, es un destino más probable a largo plazo para los plásticos expuestos, lo que resulta en pequeños fragmentos rotos. Muchos de estos materiales son menos densos que el agua de mar, y una vez que ingresan a los océanos a través de las aguas residuales costeras o de desechos de embarcaciones marinas, tienden a permanecer allí indefinidamente.
Se sabe que la quema abierta de materiales poliméricos que contienen cloro (cloruro de polivinilo, por ejemplo) libera compuestos como las dioxinas que persisten en el ambiente. La incineración en las condiciones adecuadas puede eliminar efectivamente este peligro. Los productos desechados que contienen fluorocarbonos (artículos recubiertos de teflón, algunos materiales para el cuidado personal, impermeabilizantes y antiadherentes) se descomponen en sulfonato de perfluorooctano que se ha demostrado que daña a los animales acuáticos.
Peligros para los animales
Existen dos tipos generales de peligros que los polímeros pueden introducir en el medio acuático. Uno de estos se refiere a la liberación de moléculas pequeñas que actúan como disruptores hormonales como se describió anteriormente. Está bien establecido que los pequeños animales acuáticos como los peces están siendo seriamente afectados por tales sustancias en muchos ríos y sistemas estuarinos, pero no se han identificado detalles de las fuentes e identidades de estas moléculas. Un factor de confusión es la liberación de aguas residuales que contienen drogas anticonceptivas humanas (que tienen un efecto feminizador en el desarrollo sexual) en muchas vías fluviales.
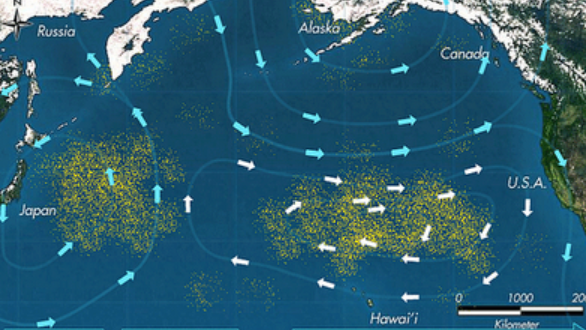
El otro peligro se relaciona con trozos de desechos plásticos que los animales acuáticos confunden con la comida o se enredan en (Figura\(\PageIndex{1}\)).


Estos peligros ocurren en todo el océano, pero se acentúan mucho en regiones conocidas como giras. Se trata de regiones del océano en las que una combinación de corrientes oceánicas impulsa vórtices permanentes que tienden a recolectar y concentrar materiales flotantes. Los más notorios de estos son los Great Pacific Gyres que han acumulado cantidades asombrosas de desechos plásticos.
. 
Recycling
The huge quantity (one estimate is 108 metric tons per year) of plastic materials produced for consumer and industrial use has created a gigantic problem of what to do with plastic waste which is difficult to incinerate safely and which, being largely non-biodegradable, threatens to overwhelm the capacity of landfills. An additional consideration is that de novo production most of the major polymers consumes non-renewable hydrocarbon resources.

Plastics recycling has become a major industry, greatly aided by enlightened trash management policies in the major developed nations. However, it is plagued with some special problems of its own:
- Recycling is only profitable when there is a market for the regenerated material. Such markets vary with the economic cycle (they practically disappeared during the recession that commenced in 2008.)
- The energy-related costs of collecting and transporting plastic waste, and especially of processing it for re-use, are frequently the deciding factor in assessing the practicability of recycling.
- Collection of plastic wastes from diverse sources and locations and their transport to processing centers consumes energy and presents numerous operational problems.
- Most recycling processes are optimized for particular classes of polymers. The diversity of plastic types necessitates their separation into different waste streams — usually requiring manual (i.e., low-cost) labor. This in turn encourages shipment of these wastes to low-wage countries, thus reducing the availability of recycled materials in the countries in which the plastics originated.
Some of the major recycling processes include
- Thermal decomposition processes that can accommodate mixed kinds of plastics and render them into fuel oil, but the large inputs of energy they require have been a problem.
- A very small number of condensation polymers can be depolymerized so that the monomers can be recovered and re-used.
- Thermopolymers can be melted and pelletized, but those of widely differing types must be treated separately to avoid incompatability problems.
- Thermosets are usually shredded and used as filler material in recycled thermopolymers.
Other processes
A process has also been developed in which many kinds of plastic can be used as a carbon source in the recycling of
scrap steel. There is also a possibility of mixed recycling of different plastics, which does not require their separation. It is called compatibilization and requires use of special chemical bridging agents compatibilizers. It can help to keep the quality of recycled material and to skip often expensive and inefficient preliminary scanning of waste plastics streams and their separation/purification.

Recycled Plastics
Seven groups of plastic polymers, each with specific properties, are used worldwide for packaging applications (see Table \(\PageIndex{1}\)). Each group of plastic polymer can be identified by its plastic identification code (PIC), usually a number or a letter abbreviation. For instance, low-density polyethylene can be identified by the number "4" or the letters "LDPE". The PIC appears inside a three-chasing-arrow recycling symbol. The symbol is used to indicate whether the plastic can be recycled into new products.
The PIC was introduced by the Society of the Plastics Industry, Inc., to provide a uniform system for the identification of various polymer types and to help recycling companies separate various plastics for reprocessing. Manufacturers of plastic products are required to use PIC labels in some countries/regions and can voluntarily mark their products with the PIC where there are no requirements. Consumers can identify the plastic types based on the codes usually found at the base or at the side of the plastic products, including food/chemical packaging and containers.
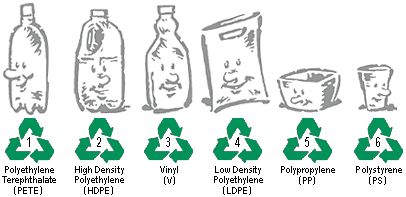
Not all categories are accepted by all local recycling authorities, so residents need to be informed about which kinds should be placed in recycling containers and which should be combined with ordinary trash.
| Plastic identification code | Type of plastic polymer | Properties | Common packaging applications | Melting temperatures (°C) |
|---|---|---|---|---|

|
Polyethylene terephthalate(PET, PETE) | Clarity, strength, toughness, barrier to gas and moisture. | Soft drink, water and salad dressing bottles; peanut butter and jam jars; ice cream cone lids; small consumer electronics | Tm = 250 |

|
High-density polyethylene(HDPE) | Stiffness, strength, toughness, resistance to moisture, permeability to gas | Water pipes, Gas & Fire Pipelines, Electrical & Communications conduit, hula hoop rings, five gallon buckets, milk, juice and water bottles; grocery bags, some shampoo/toiletry bottles | Tm = 130 |

|
Polyvinyl chloride(PVC) | Versatility, ease of blending, strength, toughness. | Blister packaging for non-food items; cling films for non-food use. May be used for food packaging with the addition of the plasticisers needed to make natively rigid PVC flexible. Non-packaging uses are electrical cable insulation; rigid piping; vinyl records. | Tm = 240 |

|
Low-density polyethylene(LDPE) | Ease of processing, strength, toughness, flexibility, ease of sealing, barrier to moisture | Frozen food bags; squeezable bottles, e.g. honey, mustard; cling films; flexible container lids | Tm = 120 |

|
Polypropylene(PP) | Strength, toughness, resistance to heat, chemicals, grease and oil, versatile, barrier to moisture. | Reusable microwaveable ware; kitchenware; yogurt containers; margarine tubs; microwaveable disposable take-away containers; disposable cups; soft drink bottle caps; plates. | Tm = 173 |

|
Polystyrene(PS) | Versatility, clarity, easily formed | Egg cartons; packing peanuts; disposable cups, plates, trays and cutlery; disposable take-away containers | Tm = 240 |

|
Other (often polycarbonateor ABS) | Dependent on polymers or combination of polymers | Beverage bottles, baby milk bottles. Non-packaging uses for polycarbonate, compact discs, "unbreakable" glazing, electronic apparatus housing, lenses (including sunglasses), prescription glasses, automotive headlamps, riot shields, instrument panels. |
Polycarbonate: Tm = 225 |
Tire Recycling
The large number of rubber tires that are disposed of, together with the increasing reluctance of landfills to accept them, has stimulated considerable innovation in the re-use of this material, especially in the construction industry.
Plastics and Fire Hazards
The term fire (or flame)-retardant as applied to organic (i.e., containing carbon) materials, is intended to refer to reduced fire hazard, as all will burn under certain circumstances. Fabric flammability is an important textile issue, especially for stage drapery that will be used in a public space such as a school, theatre or special event venue. In the United States, Federal regulations require that drapery fabrics used in such spaces be certified as flame or fire-retardant. For draperies and other fabrics used in public places, this is known as the NFPA 701 Test, which follows standards developed by the National Fire Protection Association (NFPA). Although all fabrics will burn, some are naturally more resistant to fire than others. Those that are more flammable can have their fire resistance drastically improved by treatment with fire-retardant chemicals. Inherently flame-retardant fabrics such as polyester are commonly used for flame retardant curtain fabrics.
The deaths in fiery crashes of race car drivers Fireball Roberts at Charlotte, and Eddie Sachs and Dave MacDonald at Indianapolis in 1964 led to the use of flame-resistant ics such as Nomex. Nomex and related aramid polymers are related to nylon, but have aromatic backbones, and hence are more rigid and more durable. Nomex is an example of a meta variant of the aramids (Kevlar is a para aramid). Unlike Kevlar, Nomex strands cannot align during filament polymerization and has less strength. However, it has excellent thermal, chemical, and radiation resistance for a polymer material.
A Nomex hood is a common piece of racing and firefighting equipment. It is placed on the head on top of a firefighter's face mask. The hood protects the portions of the head not covered by the helmet and face mask from the intense heat of the fire.

Wildland firefighters wear Nomex shirts and trousers as part of their personal protective equipment during wildfire suppression activities.
Racing car drivers wear driving suits constructed of Nomex and or other fire retardant materials, along with Nomex gloves, long underwear, balaclavas, socks, helmet lining and shoes, to protect them in the event of a fire.
Military pilots and aircrew wear flight suits made of over 92 percent Nomex to protect them from the possibility of cockpit fires and other mishaps. Recently, troops riding in ground vehicles have also begun wearing Nomex. Kevlar thread is often used to hold the fabric together at seams.
Military tank drivers also typically use Nomex hoods as protection against fire.
Plasticizers and Pollution
Plasticizers (UK: plasticisers) or dispersants are additives that increase the plasticity or decrease the viscosity of a material. These substances are compounded into certain types of plastics to render them more flexible by lowering the glass transition temperature. They accomplish this by taking up space between the polymer chains and acting as lubricants to enable the chains to more readily slip over each other. Many (but not all) are small enough to be diffusible and a potential source of health problems.
Polyvinyl chloride polymers are one of the most widely-plasticized types, and the odors often associated with flexible vinyl materials such as garden hoses, waterbeds, cheap shower curtains, raincoats and upholstery are testament to their ability to migrate into the environment.
 The well-known "new car smell" is largely due to plasticizer release from upholstery and internal trim.
The well-known "new car smell" is largely due to plasticizer release from upholstery and internal trim.
According to 2014 data, the total global market for plasticizers was 8.4 million metric tonnes including 1.3 million metric tonnes in Europe.
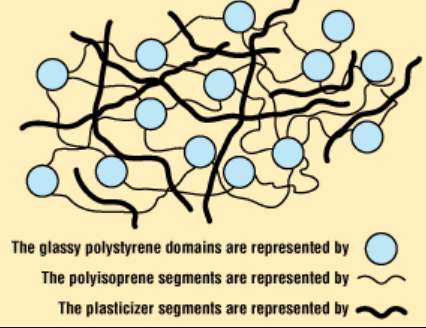
Substantial concerns have been expressed over the safety of some plasticizers, especially because some low molecular weight ortho-phthalates have been classified as potential endocrine disruptors with some developmental toxicity reported.
Summary
- Plastics are found everywhere due to its low cost, versatility, ease of use etc.
- Plastics pose a threat to the environment due to residual or degradation products that contribute to air and water pollution.
- Plastics hazards to animals and marine life as these living creatures mistake them for food.
- Plastic polymers are classified into seven groups for recycling purposes.
Contributors and Attributions
Stephen Lower, Professor Emeritus (Simon Fraser U.) Chem1 Virtual Textbook
- Wikipedia

