10.4: Los diagramas de fase
- Page ID
- 1880
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)habilidades para desarrollar
- Explicar la construcción y el uso de un diagrama de fase típico.
- Usar diagramas de fase para identificar fases estables a temperaturas y presiones dadas, y para describir las transiciones de fase resultantes de cambios en estas propiedades
- Describir la fase fluida supercrítica de la materia.
En el módulo anterior, se describió la variación de la presión de vapor de equilibrio de un líquido con la temperatura. Considerando la definición del punto de ebullición, los gráficos de presión de vapor versus temperatura representan cómo el punto de ebullición del líquido varía con la presión. También se describió el uso de curvas de calentamiento y enfriamiento para determinar el punto de fusión (o congelación) de una sustancia. Hacer tales mediciones en un amplio rango de presiones produce datos que se pueden presentar gráficamente como un diagrama de fase. Un diagrama de fase combina gráficos de presión versus temperatura para los equilibrios de transición de fase líquido-gas, sólido-líquido y sólido-gas de una sustancia. Estos diagramas indican los estados físicos que existen bajo condiciones específicas de presión y temperatura, y también proporcionan la dependencia de la presión de las temperaturas de transición de fase (puntos de fusión, puntos de sublimación, puntos de ebullición). Un diagrama de fase típico para una sustancia pura se muestra en la Figura \(\PageIndex{1}\).

Para ilustrar la utilidad de estos gráficos, considere el diagrama de fase para el agua que se muestra en la Figura \(\PageIndex{2}\).

Podemos usar el diagrama de fases para identificar el estado físico de una muestra de agua en condiciones específicas de presión y temperatura. Por ejemplo, una presión de 50 kPa y una temperatura de −10 °C corresponden a la región del diagrama etiquetada como "hielo". En estas condiciones, el agua existe solo como un sólido (hielo). Una presión de 50 kPa y una temperatura de 50 °C corresponden a la región "agua"; aquí, el agua existe solo como un líquido. A 25 kPa y 200 °C, el agua existe solo en estado gaseoso. Tenga en cuenta que en el diagrama de fase H2O, los ejes de la presión y la temperatura no están dibujados a una escala constante para permitir la ilustración de varias características importantes como se describe aquí.
La curva BC en la Figura \(\PageIndex{2}\) es la gráfica de presión de vapor versus temperatura como se describe en el módulo anterior de este capítulo. Esta curva de "líquido-vapor" separa las regiones líquida y gaseosa del diagrama de fases y proporciona el punto de ebullición del agua a cualquier presión. Por ejemplo, a 1 atm, el punto de ebullición es de 100 °C. Observe que la curva de líquido-vapor termina a una temperatura de 374 °C y una presión de 218 atm, lo que indica que el agua no puede existir como líquido por encima de esta temperatura, independientemente de la presión. Las propiedades físicas del agua en estas condiciones son intermedias entre las de sus fases líquida y gaseosa. Este estado único de la materia se llama el fluido supercrítico, un tema que se describirá en la siguiente sección de este módulo.
La curva de vapor sólido, etiquetada AB en la Figura \(\PageIndex{2}\), indica las temperaturas y presiones a las cuales el hielo y el vapor de agua están en equilibrio. Estos pares de datos de temperatura-presión corresponden a los puntos de sublimación o deposición del agua. Si pudiéramos acercarnos a la línea de gas sólido en la Figura \(\PageIndex{2}\), veríamos que el hielo tiene una presión de vapor de aproximadamente 0.20 kPa a -10 °C. Por lo tanto, si colocamos una muestra congelada en el vacío con una presión mas baja de 0.20 kPa, el hielo sublimará. Esta es la base del proceso de la "liofilización" que se usa a menudo para conservar alimentos, como el helado que se muestra en la Figura \(\PageIndex{3}\).

La curva de sólido-líquido etiquetada BD muestra las temperaturas y las presiones a las que el hielo y el agua líquida están en equilibrio, representando los puntos de fusión/congelación del agua. Tenga en cuenta que esta curva exhibe un poco de una pendiente negativa (muy exagerada para mayor claridad), lo que indica que el punto de fusión del agua disminuye un poco a medida que aumenta la presión. El agua es una sustancia inusual a este respecto, ya que la mayoría de las sustancias exhiben un aumento en el punto de fusión al aumentar la presión. Este comportamiento es en parte responsable del movimiento de los glaciares, como el que se muestra en la Figura \(\PageIndex{4}\). El fondo de un glaciar experimenta una presión inmensa debido a su peso que puede derretir parte del hielo, formando una capa de agua líquida sobre la cual el glaciar puede deslizarse más fácilmente.

El punto de intersección de las tres curvas se etiqueta B en la Figura \(\PageIndex{2}\). A la presión y la temperatura representadas por este punto, las tres fases del agua coexisten en equilibrio. Este par de datos de temperatura-presión se llama el punto triple. A presiones mas bajas del punto triple, el agua no puede existir como líquido, independientemente de la temperatura.
Ejemplo \(\PageIndex{1}\): Determinando el Estado De fase Del Agua
Usando el diagrama de fase para el agua dado en la Figura 10.4.2, determine el estado del agua a las siguientes temperaturas y presiones:
- −10 °C y 50 kPa
- 25 °C y 90 kPa
- 50 °C y 40 kPa
- 80 °C y 5 kPa
- −10 °C y 0.3 kPa
- 50 °C y 0.3 kPa
Solución
Usando el diagrama de fase para el agua, podemos determinar que el estado del agua a cada temperatura y presión dada es la siguiente: (a) sólido; (b) líquido; (c) líquido; (d) gas; (e) sólido; (f) gas.
Ejercicio \(\PageIndex{1}\)
¿Qué cambios de fase puede experimentar el agua a medida que cambia la temperatura si la presión se mantiene a 0.3 kPa? Si la presión se mantiene a 50 kPa?
- Respuesta:
-
A 0.3 kPa: s⟶ g a −58 °C. A 50 kPa: s⟶ l a 0 °C, l ⟶ g a 78 °C
Considere el diagrama de fase para el dióxido de carbono que se muestra en la Figura \(\PageIndex{5}\) como otro ejemplo. La curva sólido-líquido exhibe una pendiente positiva, lo que indica que el punto de fusión del CO2 aumenta con la presión como lo hace con la mayoría de las sustancias (el agua es una notable excepción como se describió anteriormente). Observe que el punto triple está muy por encima de 1 atm, lo que indica que el dióxido de carbono no puede existir como líquido en condiciones de presión ambiente. En cambio, enfriando el dióxido de carbono gaseoso a 1 atm resulta en su deposición en el estado sólido. Del mismo modo, el dióxido de carbono sólido no se funde a una presión de 1 atm sino que se sublima para producir el CO2 gaseoso. Finalmente, observe que el punto crítico para el dióxido de carbono se observa a una temperatura y presión relativamente moderadas en comparación con el agua.

Ejemplo \(\PageIndex{2}\): Determinando el estado de fase del dióxido de carbono
Usando el diagrama de fase para el dióxido de carbono que se muestra en la Figura 10.4.5, determine el estado del CO2 a las siguientes temperaturas y presiones:
- −30 °C y 2000 kPa
- −60 °C y 1000 kPa
- −60 °C y 100 kPa
- 20 °C y 1500 kPa
- 0 °C y 100 kPa
- 20 °C y 100 kPa
Solución
Usando el diagrama de fase para el dióxido de carbono proporcionado, podemos determinar que el estado de CO2 a cada temperatura y presión dada es el siguiente: (a) líquido; (b) sólido; (c) gas; (d) líquido; (e) gas; (f) gas.
Ejercicio \(\PageIndex{2}\)
¿Determine los cambios de fase que sufre el dióxido de carbono cuando se varía su temperatura, manteniendo así su presión constante a 1500 kPa? ¿A 500 kPa? ¿A cuál temperatura aproximadamente ocurren estos cambios de fase?
- Respuesta
-
a 1500 kPa: s⟶ l a −45 °C, l⟶ g a −10 °C; a 500 kPa: s⟶ g a −58 °C
Fluidos Supercríticos
Si colocamos una muestra de agua en un recipiente sellado a 25°C, eliminamos el aire y dejamos que se establezca el equilibrio de vaporización-condensación, nos queda una mezcla de agua líquida y vapor de agua a una presión de 0.03 atm. Se observa claramente un límite distintivo entre el líquido más denso y el gas menos denso. A medida que aumentamos la temperatura, aumenta la presión del vapor de agua, como se describe en la curva de líquido-gas en el diagrama de fase del agua (Figura \(\PageIndex{2}\)), y un equilibrio de dos fases de líquido y quedan las fases gaseosas. A una temperatura de 374°C, la presión de vapor ha aumentado a 218 atm, y cualquier aumento adicional en la temperatura resulta en la desaparición del límite entre las fases líquida y de vapor. Toda el agua en el recipiente ahora está presente en una sola fase cuyas propiedades físicas son intermedias entre las de los estados gaseoso y líquido. Esta fase de la materia se llama el fluido supercrítico, y la temperatura y la presión por encima de las cuales existe esta fase es el punto crítico (Figura \(\PageIndex{5}\)). Por encima de su temperatura crítica, un gas no se puede formar en liquido sin importar cuánta presión se aplique. La presión requerida para licuar un gas a su temperatura crítica se llama la presión crítica. Las temperaturas y las presiones críticas de algunas sustancias comunes se dan en la Tabla \(\PageIndex{1}\).
| Sustancia | Temperatura Crítica (K) | Presión Crítica (atm) |
|---|---|---|
| hidrógeno | 33.2 | 12.8 |
| nitrógeno | 126.0 | 33.5 |
| oxígeno | 154.3 | 49.7 |
| dióxido de carbono | 304.2 | 73.0 |
| amoniaco | 405.5 | 111.5 |
| dióxido de azufre | 430.3 | 77.7 |
| agua | 647.1 | 217.7 |
Al igual que un gas, un fluido supercrítico se expandirá y llenará un recipiente, pero su densidad es mucho mayor que las densidades de gases típicos, típicamente su densidad es cerca de las de los líquidos. Similar a los líquidos, estos fluidos son capaces de disolver los solutos no volátiles. Sin embargo, exhiben casi ninguna tensión superficial y viscosidades muy bajas, por lo que pueden penetrar más eficazmente aberturas muy pequeñas en una mezcla sólida y eliminar componentes solubles. Estas propiedades hacen que los fluidos supercríticos sean disolventes extremadamente útiles para una amplia gama de aplicaciones. Por ejemplo, el dióxido de carbono supercrítico se ha convertido en un solvente muy popular en la industria alimentaria, ya que se usa para descafeinar el café, eliminar las grasas de las papas fritas y extraer los compuestos aromatizantes y aromatizantes de los aceites cítricos. No es tóxico, es relativamente económico y no se considera contaminante. Después del uso, el CO2 se puede recuperar fácilmente reduciendo la presión y recogiendo el gas resultante.
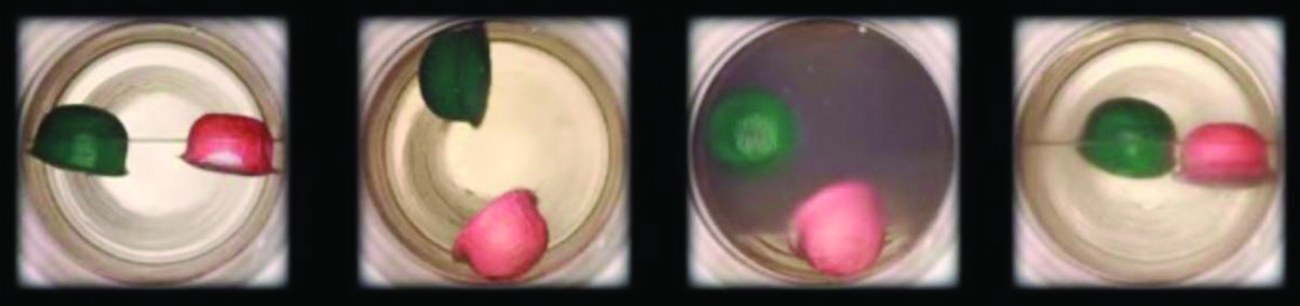
Ejemplo \(\PageIndex{3}\): La temperatura crítica del dióxido de carbono
Si sacudimos un extintor de incendios de dióxido de carbono en un día frío (18 °C), podemos escuchar el CO2 líquido chapoteando dentro del cilindro. Sin embargo, el mismo cilindro parece no contener líquido en un día caluroso de verano (35 °C). Explique estas observaciones.
Solución
En un día frío, la temperatura del CO2 está por debajo de la temperatura crítica de CO2, 304 K o 31°C (Tabla \(\PageIndex{1}\)), por lo que el CO2 líquido está presente en el cilindro. En los días calurosos, la temperatura del CO2 es mayor que su temperatura crítica de 31 °C. Por encima de esta temperatura, ninguna cantidad de presión puede licuar el CO2, por lo que no existe CO2 líquido en el extintor de incendios.
Ejercico \(\PageIndex{3}\)
El amoníaco se puede licuar por compresión a temperatura ambiente; el oxígeno no se puede licuar bajo estas condiciones. ¿Por qué los dos gases exhiben un comportamiento diferente?
- Respuesta
-
La temperatura crítica del amoníaco es de 405.5 K, que es más alta que la temperatura ambiente. La temperatura crítica del oxígeno está por debajo de la temperatura ambiente; por lo tanto, el oxígeno no se puede licuar a temperatura ambiente.
Descafeinando el café usando el CO2 supercrítico
El café es el segundo producto más comercializado del mundo, solo después del petróleo. En todo el mundo, a la gente le encanta el aroma y el sabor del café. Muchos de nosotros también dependemos de un componente del café, la cafeína, para ayudarnos a ponernos en marcha por la mañana o estar alertas por la tarde. Pero al final del día, el efecto estimulante del café puede evitar que duerma, por lo que puede optar por tomar café descafeinado por la noche.
Desde principios de 1900, se han usado muchos métodos para descafeinar el café. Todos tienen ventajas y desventajas, y todos dependen de las propiedades físicas y químicas de la cafeína. Debido a que la cafeína es una molécula algo polar, se disuelve bien en el agua, un líquido polar. Sin embargo, dado que muchos de los otros 400 compuestos que contribuyen al sabor y aroma del café también se disuelven en H2O, los procesos de descafeinado con agua caliente también pueden eliminar algunos de estos compuestos, lo que afecta negativamente el olor y el sabor del café descafeinado. El diclorometano (CH2Cl2) y el acetato de etilo (CH3CO2C2H5) tienen una polaridad similar a la cafeína y, por lo tanto, son disolventes muy efectivos para la extracción de cafeína, pero ambos también eliminan algunos componentes de sabor y aroma, y su uso requiere largos tiempos de extracción y limpieza. Debido a que ambos solventes son tóxicos, se han planteado problemas de salud con respecto al efecto del solvente residual que queda en el café descafeinado.
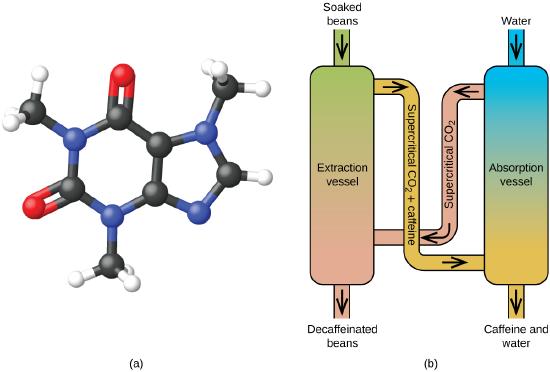
La extracción de fluidos supercríticos con el dióxido de carbono ahora se está usando ampliamente como un método de descafeinar el café más eficaz y respetuoso con el medio ambiente (Figura \(\PageIndex{7}\)). A temperaturas superiores a 304.2 K y presiones superiores a 7376 kPa, el CO2 es un fluido supercrítico, con propiedades de un gas y un líquido. Como un gas, penetra profundamente en los granos de café; como un líquido, disuelve efectivamente ciertas sustancias. La extracción supercrítica del dióxido de carbono de los granos de café al vapor elimina el 97-99% de la cafeína, dejando intactos los compuestos de aroma y sabor del café. Debido a que el CO2 es un gas en condiciones estándar, su eliminación de los granos de café extraídos se realiza fácilmente, al igual que la recuperación de la cafeína del extracto. La cafeína recuperada de los granos de café a través de este proceso es un producto valioso que después se puede usar como un aditivo para otros alimentos o drogas.
Resumen
Las condiciones de temperatura y presión a las que existe una sustancia en estado sólido, líquido y gaseoso se resumen en un diagrama de fase para esa sustancia. Los diagramas de fase son gráficos combinados de tres curvas de equilibrio presión-temperatura: sólido-líquido, líquido-gas y sólido-gas. Estas curvas representan las relaciones entre las temperaturas y las presiones de transición de fase. El punto de intersección de las tres curvas representa el punto triple de la sustancia: la temperatura y la presión a las que las tres fases están en equilibrio. A presiones por debajo del punto triple, una sustancia no puede existir en estado líquido, independientemente de su temperatura. El término de la curva líquido-gas representa el punto crítico de la sustancia, la presión y la temperatura por encima de las cuales no puede existir una fase líquida.
Glosario
- punto crítico
- temperatura y presión por encima de las cuales un gas no puede condensarse en un líquido
- diagrama de fases
- gráfico de presión-temperatura que resume las condiciones bajo las cuales pueden existir las fases de una sustancia
- fluido supercrítico
- sustancia a una temperatura y presión superiores a su punto crítico; exhibe propiedades intermedias entre las de los estados gaseoso y líquido
- triple punto
- temperatura y presión a la que las fases de vapor, líquido y sólido de una sustancia están en equilibrio
Contribuyentes
Paul Flowers (Universidad de Carolina del Norte - Pembroke), Klaus Theopold (Universidad de Delaware) y Richard Langley (Stephen F. Austin Universidad del Estado) con autores contribuyentes. Contenido del libro de texto producido por la Universidad de OpenStax tiene licencia de Atribución de Creative Commons Licencia 4.0 licencia. Descarge gratis en http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110)."
Ana Martinez (amartinez02@saintmarys.edu) contribuyó a la traducción de este texto.


