10.5: Problems for Chapter 10
- Page ID
- 2388
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Link to Solution Manual
P10.1: Show a mechanism for the following kinase reaction (it is appropriate to abbreviate ATP and ADP, but be sure to draw out all the bonds where the chemical action is taking place). Draw the pentavalent transition state / intermediate in three dimensions.
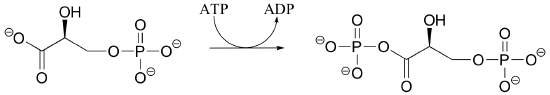
P10.2: Draw a mechanism for reaction catalyzed by shikimate kinase (in the aromatic amino acid biosynthesis pathway). Stereochemistry in the product is not indicated in the figure below - in your mechanism, show the stereochemistry of the product, and explain how you are able to predict it from your knowledge of kinase reactions.
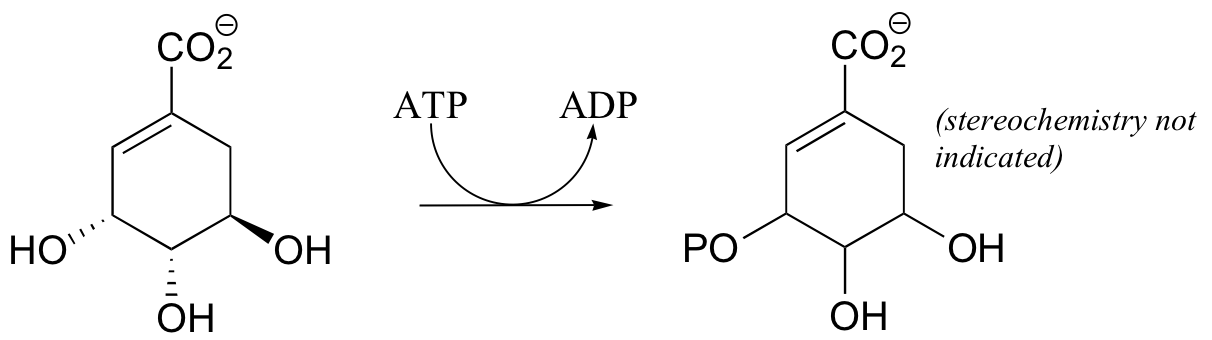
P10.3: Draw a complete mechanism for the following kinase reaction (in the ribonucleotide biosynthetic pathway)
P10.4: Draw a mechanism for the hydrolysis of phosphoserine to serine. Draw the pentavalent transition state / intermediate in three dimensions.
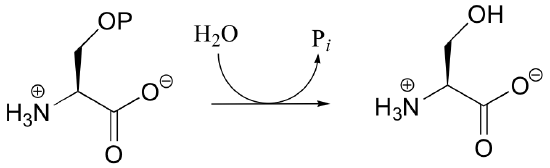
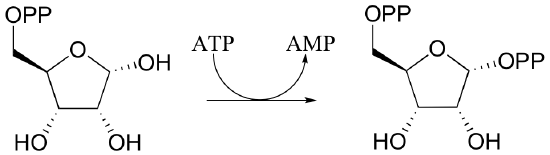
P10.5: Draw mechanisms for the following kinase reactions of ribonucleotide biosynthesis.
a)
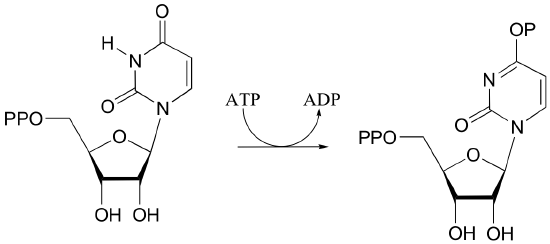
b)
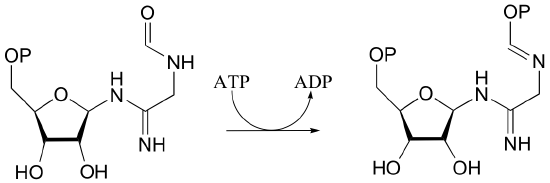
P10.6: Draw a mechanism for the following reaction.
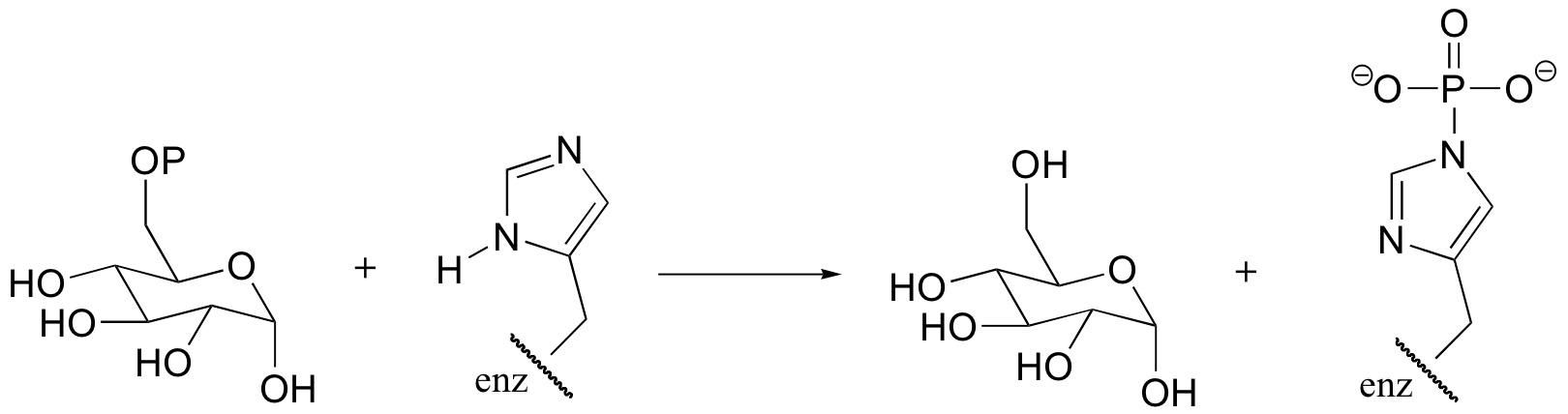
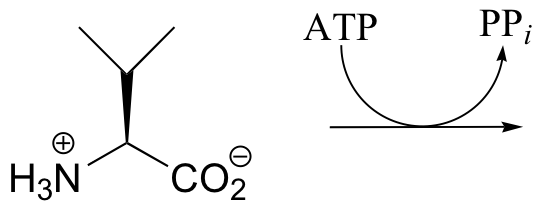
P10.7: The carboxylate of valine is transformed by an ATP-dependent reaction as an early step in the biosynthesis of the antibiotic penicillin. Predict the product of this reaction, and draw a mechanism for its formation.
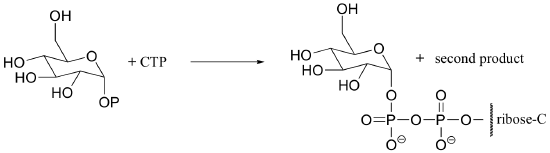
P10.8: The reaction below is an early step in the synthesis of tyvelose, a sugar found on the surface of some pathogenic bacteria. Show a mechanism for the reaction, and indicate the second product that is released by the enzyme.
P10.9:
a) Propose a mechanism for the following hydrolysis of GDP-glucose. Is this a typical phosphoryl group transfer mechanism? Explain.
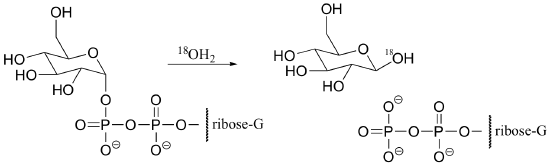
b) If the reaction above proceeded by a typical phosphoryl transfer mechanism, draw the products that would be expected (be sure to show the location of any 18O atoms).
P10.10: (This question assumes a basic knowledge of DNA structure and the idea of supercoiling). DNA topoisomerase enzymes catalyze the temporary 'nicking' of one strand of double-stranded DNA, which allows supercoilded DNA to 'unwind' before the nicked strand is re-ligated. During the unwinding process, the 3' end of the nicked strand is transferred to a tyrosine in the enzyme's active site, effectively holding it in place while the 5' end rotates. Overall, the stereochemical configuration of the bridging phosphate is retained. Propose a mechanism for this nicking and re-ligating process.
P10.11:
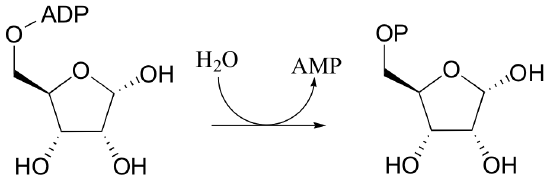
Propose two possible mechanisms for the following hydrolysis reaction, then suggest an isotopic labeling experiment to discern the two.
P10.12: Imagine a generic nucleophile, which we will refer to simply as 'Nu', that is free to attack any of the three phosphate groups of ATP, as well as the 5' carbon of the ribose group.
- Draw all the products that would result from all possible such attacks on ATP.
- There is an actual example in this text of all but one of these possible pathways. Which pathway is there no example for? List one example for each of the remaining pathways (hint - you will need to look in Chapter 9 for one example, and ahead at Chapter 11 for another !)
Challenge Problems
C10.1: The most important physiological role of the enzyme glucose-6-phosphatase is to catalyze the transfer of the phosphate from glucose-6-phosphate to water. This reaction is essentially irreversible from a thermodynamic standpoint (recall that phosphorylation of glucose required ATP as the phosphate donor).
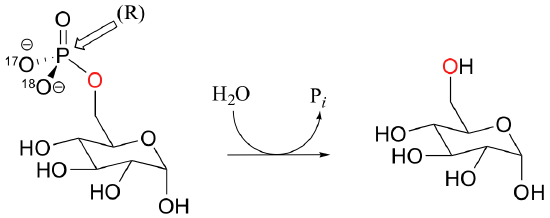
However, it is also capable of catalyzing the transfer of the phosphate group from glucose-6-phosphate directly to another glucose molecule. Because products are the same as the starting compounds, this reaction is of course highly reversible.
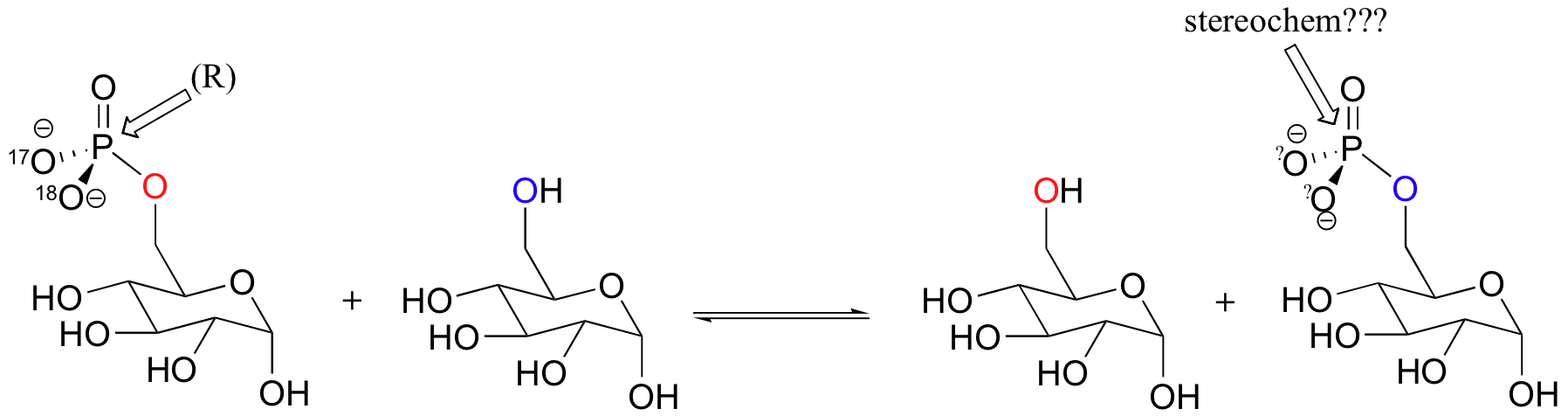
Glucose-phosphate, with 17O and 18O labels on the phosphate group in the (R) configuration, was incubated with enzyme and glucose long enough for the reaction to reach equilibrium. The glucose-6-phosphate present was then isolated and the stereochemical configuration of the phosphate group determined. Explain what you would expect to observe if a) the phosphate transfer occurred directly, in a single step, between the two glucose molecules, or b) the transfer occurred in two steps, with an intermediate in which an active site enzyme residue was phosphorylated. Specifically, what would you observe for each case in terms of the stereochemistry at the phosphate group?
C10.2: In the carboxylation reaction shown below, two possible mechanisms have been suggested. The first involves a concerted first step to form intermediate I:
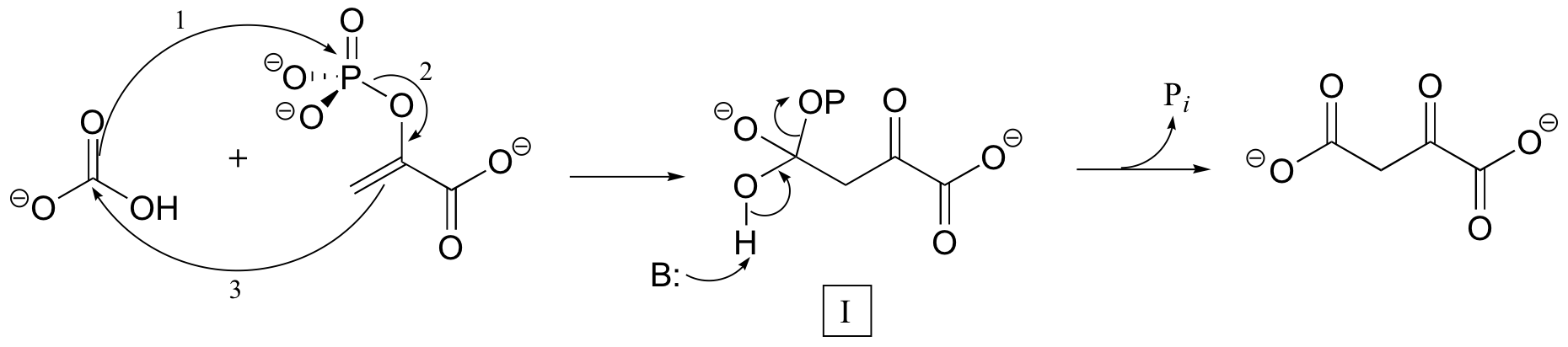
Alternatively, the mechanism could occur in two separate steps, with the phosphoryl transfer indicated by arrows 1 and 2 occurring first, followed by formation of the new carbon-carbon bond (arrow 3) to form intermediate I.
In order to explore these two possibilities, the reaction was run with an alternate substrate in which two of the non-bridging oxygens in the phosphate group were replaced with 17O, and sulfur, creating a chiral phosphorus center with the configuration shown below.
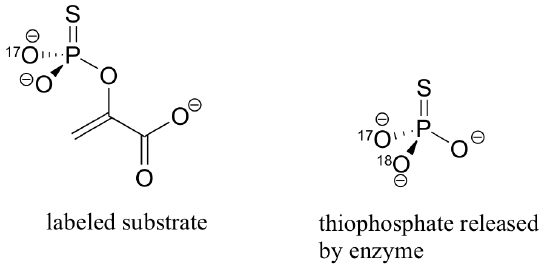
Also, the reaction was run in H218O, with fully 18O-labeled bicarbonate (HC18O3-).
The inorganic thiophosphate (PO2S3-) released from this reaction was found to have the configuration indicated above. What does this indicate about the concerted or stepwise nature of the reaction that results in the formation of intermediate B? Explain your answer in detail. Hint: it will be very helpful to construct and compare models of the possible transition states / reactive intermediates involved.
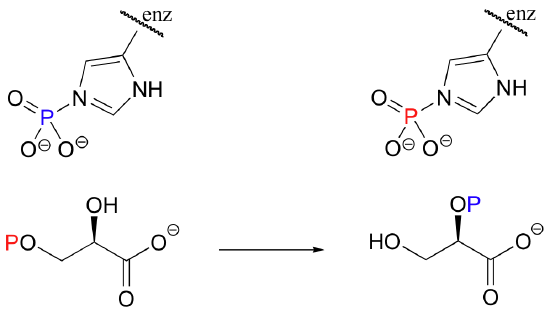
C10.3: Isomerization of 3-phosphoglycerate to 3-phosphoglycerate (a reaction in glycolysis) has been shown to occur with the participation of a phosphorylated histidine residue in the enzyme's active site. The two phosphorus atoms are distinguished in the reaction below by color. With this information, propose a mechanism for the reaction.