7.8: Problems for Chapter 7
( \newcommand{\kernel}{\mathrm{null}\,}\)
Link to Solution Manual
P7.1: In an enzyme, an Asp side chain is surrounded by phenylalanine, alanine, tryptophan, and leucine residues. Another Asp side chain located on the surface of the protein, pointing out into the surrounding water. Which residue has the higher pKa, and why?
P7.2: (a-d) How would the immediate proximity of a magnesium ion affect the pKa of the side chains of the following amino acids (relative to the ‘typical’ pKa values given in the text)? Assume that all residues are located in the interior of the protein structure, not in direct contact with the outside buffer solution.
- a glutamate residue?
- a lysine residue?
- a histidine residue?
- a tyrosine residue?
- How would contact with a magnesium ion effect the pKa of a bound water molecule in the interior of a protein?
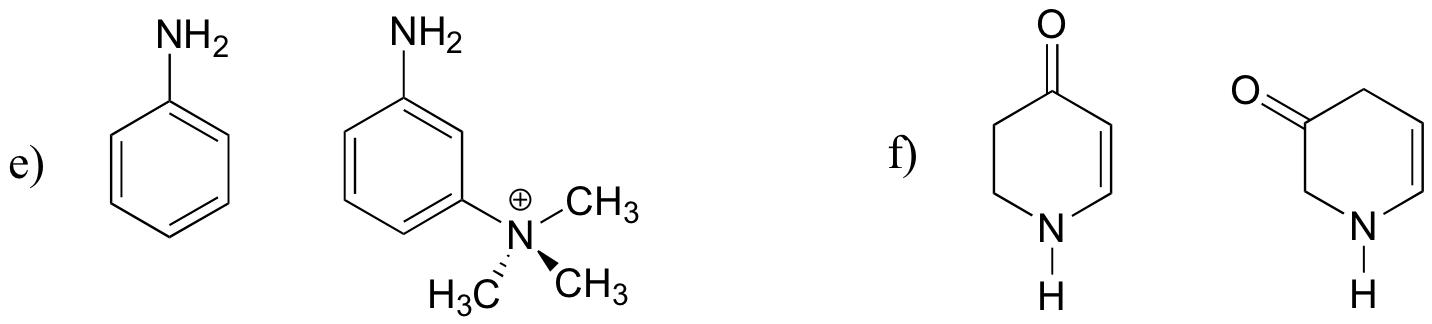
P7.3: For each pair of molecules below, choose the stronger base.

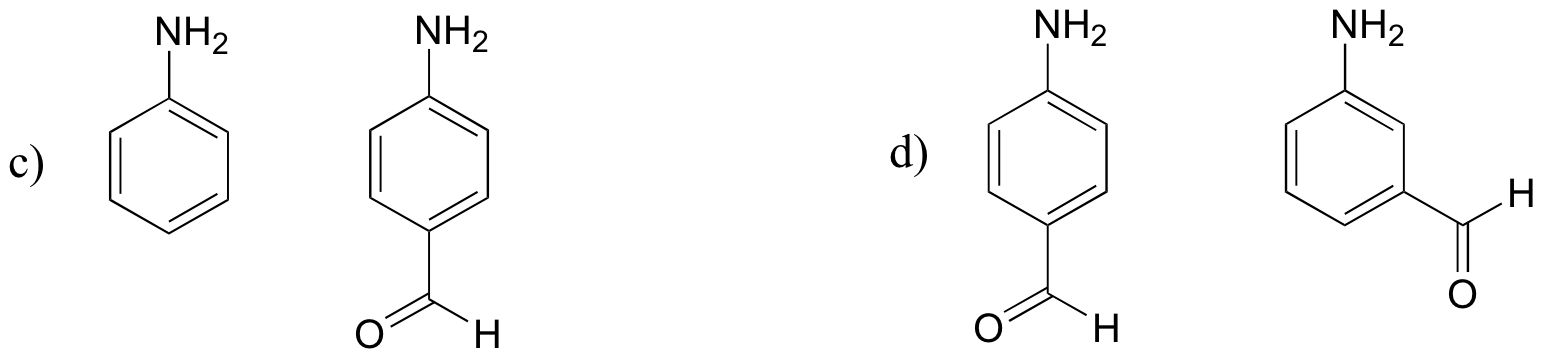
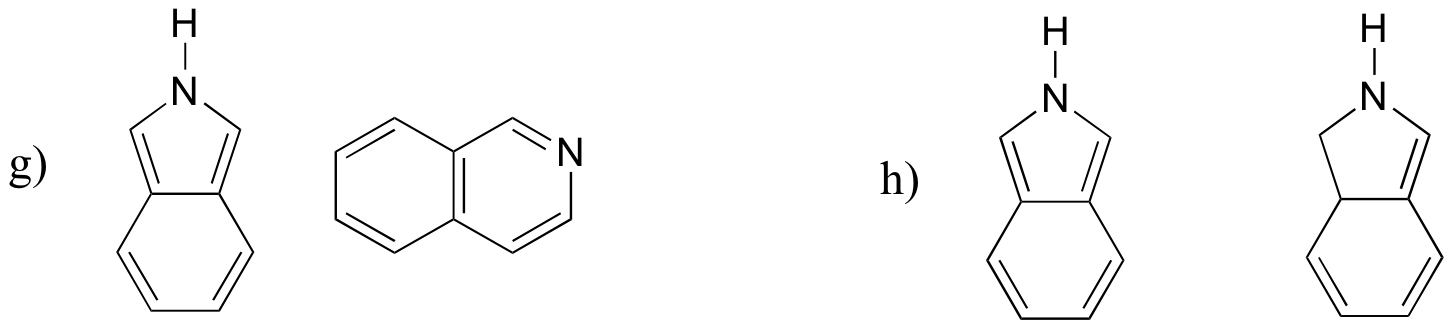

P7.4: The side chain of lysine has a pKa of approximately 10.5, while the pKa of the arginine side chain is approximately 12.5. Use resonance structures to rationalize this difference.
P7.5: The a-protons of ketones are, in general, significantly more acidic than those of esters. Account for this observation using structural arguments.
P7.6:
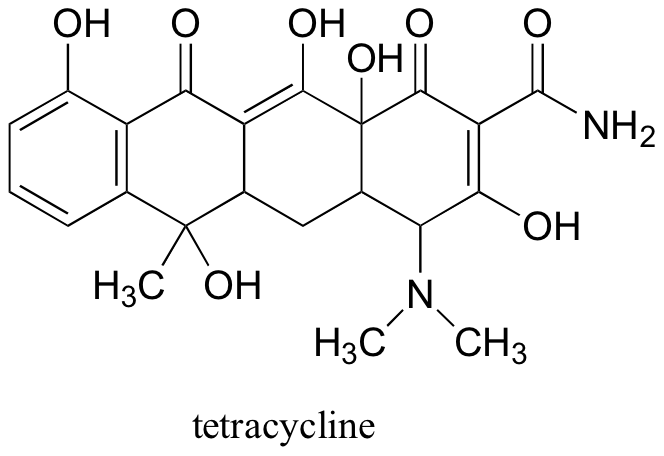
a) Locate the most acidic proton on the antibiotic tetracycline, and explain your choice.
b) Draw the structure of the conjugate base of tetracycline that has reacted with two molar equivalents of a strong base.
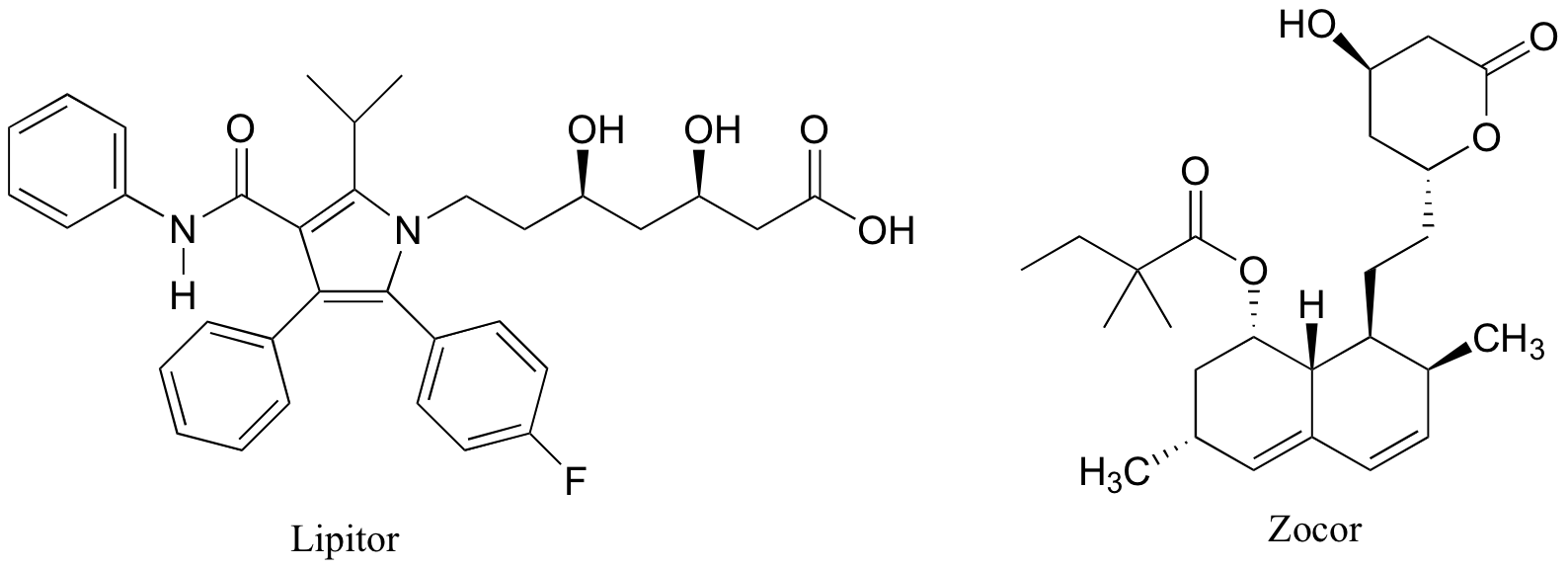
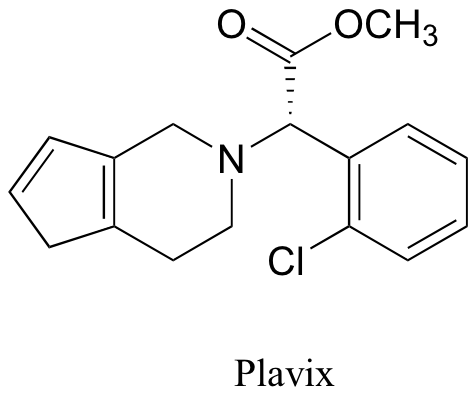
P7.7: Below are the structures of some well-known drugs.
a) What is the most acidic proton on Lipitor? What is its approximate pKa value?
b) What is the second most acidic proton on Lipitor?
c) What is the most acidic proton on Zocor? What is its approximate pKa value?
d) What is the most acidic proton on Plavix? What is its approximate pKa value?
e) Where is the most basic site on Plavix?
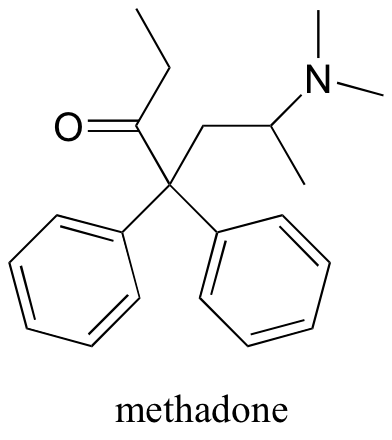
P7.8: Draw structures for the conjugate acid and the conjugate base of methadone, an opiate used in the treatment of heroin addiction.
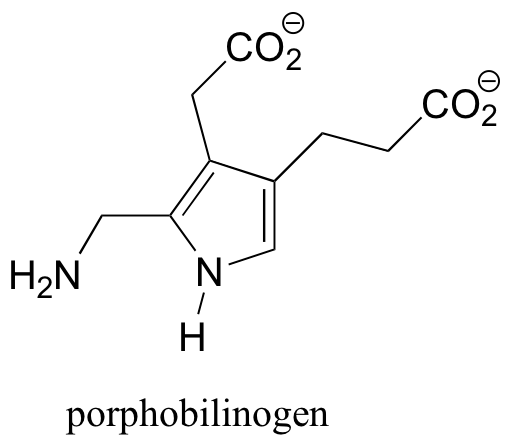
P7.9: Porphobilinogen is a precuror to the biosynthesis of chlorophyll and many other biological molecules. One of the nitrogen atoms is basic, the other is not. Which is which? Explain your reasoning.
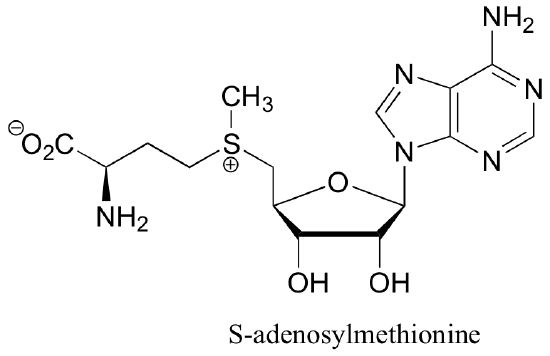
P7.10: Classify each of the amine groups in S-adenosylmethionine as an alkyl amine, aryl amine, ‘pyridine-like’ amine, or ‘pyrrole-like’ amine. Which is most basic? Which is least basic?
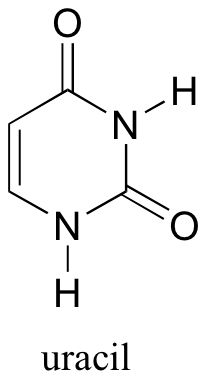
P7.11: Locate the most acidic proton on the RNA base uracil, and use resonance structures of conjugate bases to explain your reasoning.
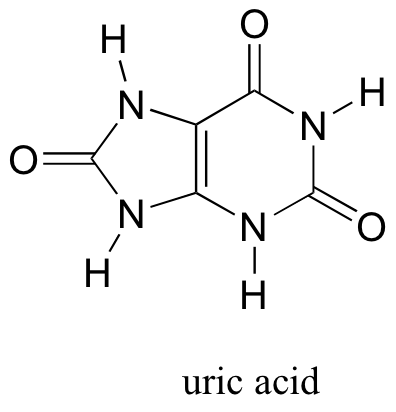
P7.12: Uric acid, an intermediate in the catabolism (breakdown) of the nucleotide adenosine, has four protons. Which is the least acidic? Use resonance structures to explain your reasoning.
P7.13: Estimate the total charge on a peptide with the sequence P-E-P-T-I-D-E (single-letter amino acid code), when it is dissolved in a buffer with pH = 7.3 (don’t forget to consider the terminal amino and carboxylate groups).
P7.14: Estimate the total charge on a dipeptide of sequence D-I.
a) in a buffer with pH = 4.0
b) in a buffer with pH = 7.3
c) in a buffer with pH = 9.6
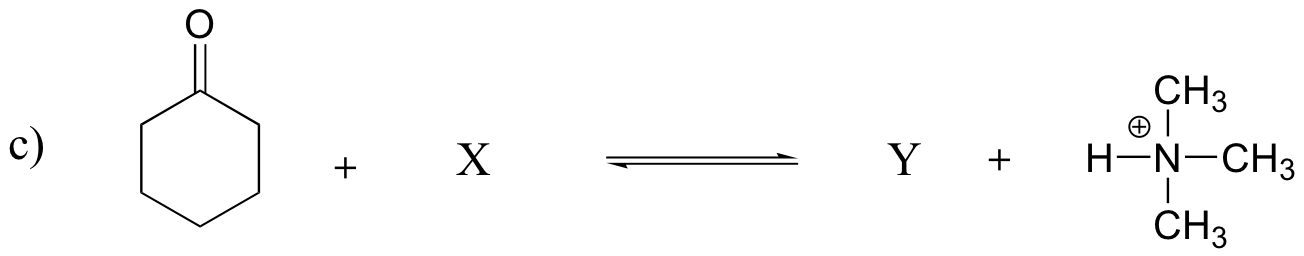
P7.15: Show the structures of species X and Y in the following acid-base reactions, and estimate the value of Keq using the pKa table. Assume that reactions involve equimolar amounts of acid and base.
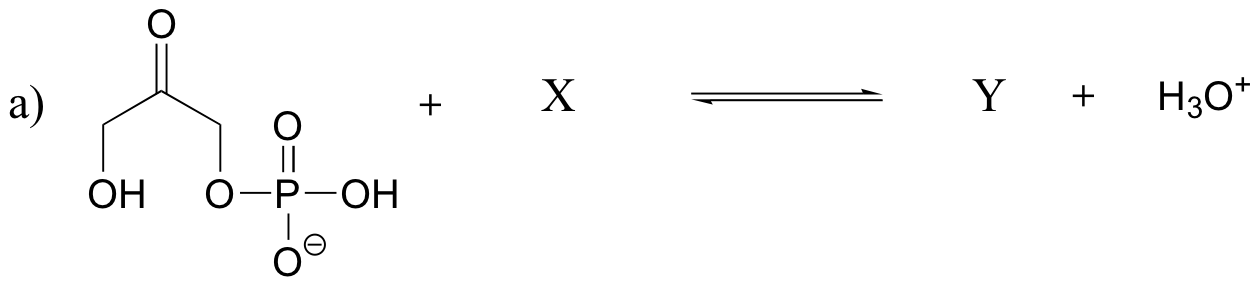
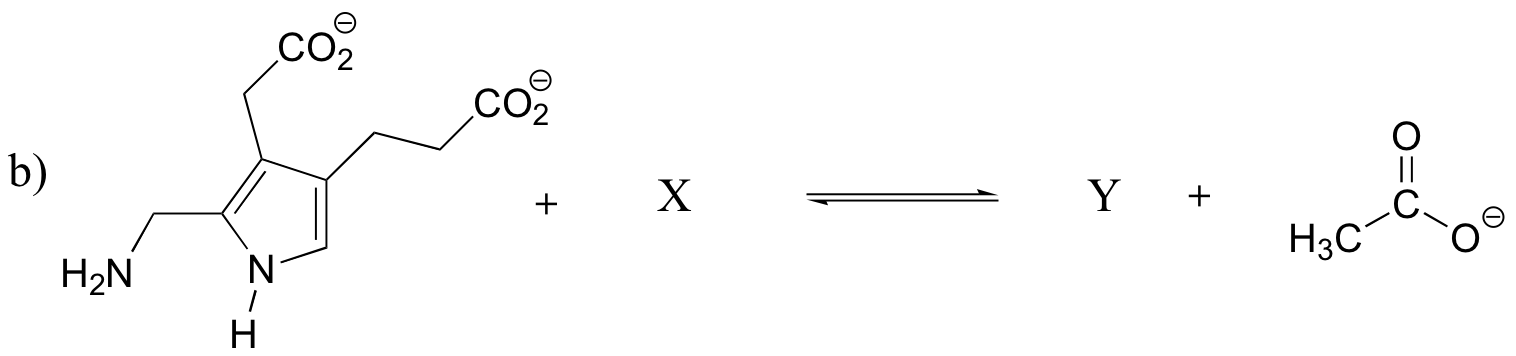
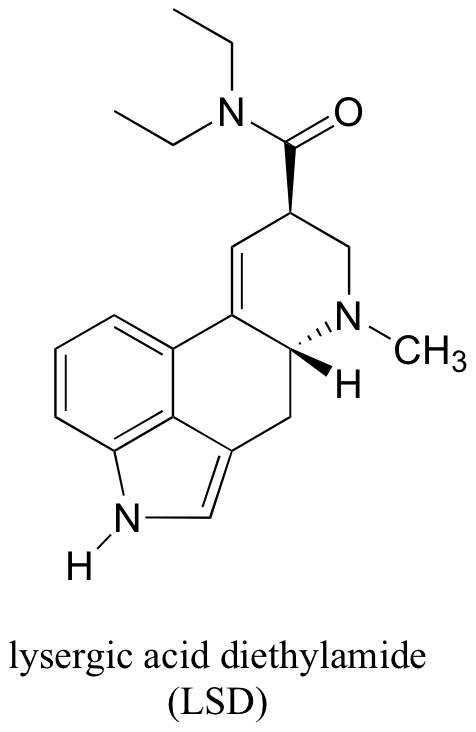
P7.16: Locate the most basic site on the structure of the hallucinogenic drug known as LSD.
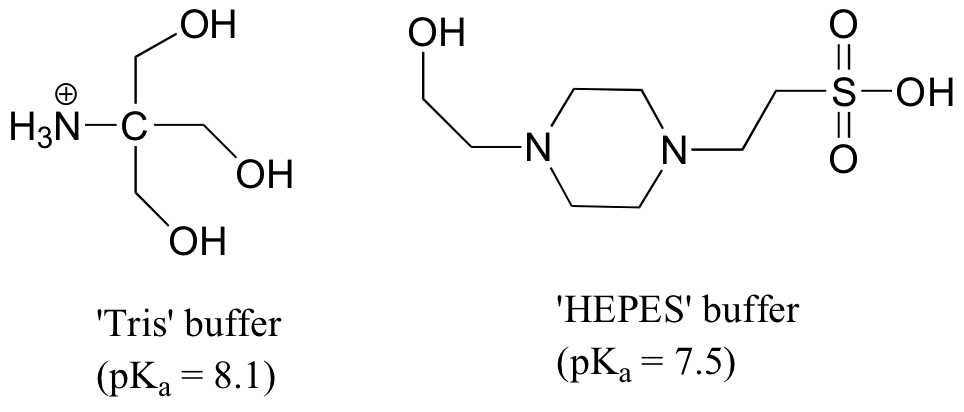
P7.17: Aqueous solutions of 'Tris' and 'HEPES' are very commonly used as buffers in biochemistry and molecular biology laboratories. You make two buffer solutions: One is 50 mM Tris at pH 7.0, the other 50 mM HEPES at pH 7.0. For each solution, calculate the concentration of buffer molecules that are in their charged (ionic) protonation states. Hint - what do you predict is this most acidic proton on HEPES?