16.11: Constantes de disociación ácida
- Page ID
- 75502
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)La siguiente tabla proporciona los valores de p K a y K a para ácidos débiles seleccionados. Todos los valores son de Martell, A. E.; Smith, R. M. Constantes de Estabilidad Crítica, Vols. 1—4. Plenum Press: Nueva York, 1976. A menos que se indique lo contrario, los valores son para 25 o C y para fuerza iónica cero. Esos valores entre paréntesis se consideran menos confiables.
Los ácidos débiles están ordenados alfabéticamente por los nombres de los compuestos neutros de los que se derivan. En algunos casos —como el ácido acético— el compuesto es el ácido débil. En otros casos, como para el ión amonio, el compuesto neutro es la base conjugada. Se muestran fórmulas químicas o fórmulas estructurales para el ácido débil completamente protonado. Se proporcionan constantes sucesivas de disociación ácida para los ácidos débiles polipróticos; donde hay ambigüedad, se identifica el protón ácido específico.
Para encontrar el valor de K b para una base débil conjugada, recuerde que
\[K_\text{a} \times K_\text{b} = K_\text{w} \nonumber\]
para un ácido débil conjugado, HA, y su base débil conjugada, A —.
| compuesto | ácido conjugado | p K a | K a |
|---|---|---|---|
| ácido acético | \(\ce{CH3COOH}\) | 4.757 | \(1.75 \times 10^{-5}\) |
| ácido adípico | 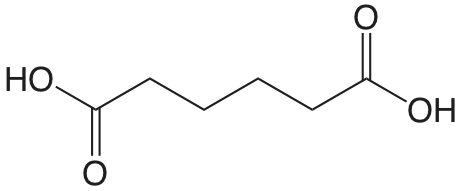 |
4.42 5.42 |
\(3.8 \times 10^{-5}\) \(3.8 \times 10^{-6}\) |
| alanina | 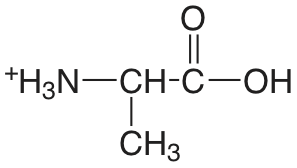 |
2.348 (\(\ce{COOH}\)) 9.867 (\(\ce{NH3}\)) |
\(4.49 \times 10^{-3}\) \(1.36 \times 10^{-10}\) |
| aminobenceno | 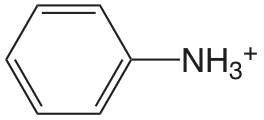 |
4.601 | \(2.51 \times 10^{-5}\) |
| Ácido 4-aminobenceno sulfónico | 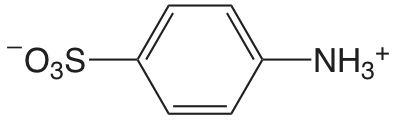 |
3.232 | \(5.86 \times 10^{-4}\) |
| Ácido 2-aminbenzoico | 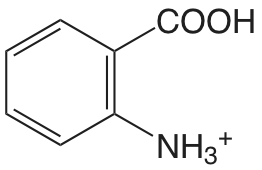 |
2.08 (\(\ce{COOH}\)) 4.96 (\(\ce{NH3}\)) |
\(8.3 \times 10^{-3}\) \(1.1 \times 10^{-5}\) |
| 2-aminofenol (\(T = 20 \text{°C}\)) | 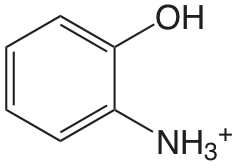 |
4.78 (\(\ce{NH3}\)) 9.97 (OH) |
\(1.7 \times 10^{-5}\) \(1.05 \times 10^{-10}\) |
| amoníaco | \(\ce{NH4+}\) | 9.244 | \(5.70 \times 10^{-10}\) |
| arginina | 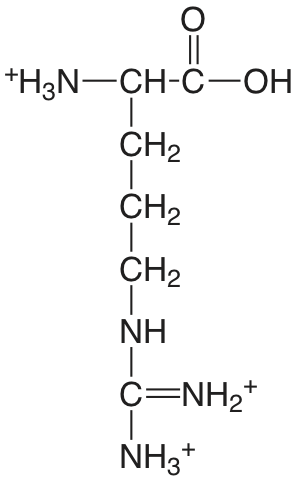 |
1.823 (COOH) 8.991 (\(\ce{NH3}\)) [12.48] (\(\ce{NH2}\)) |
\(1.50 \times 10^{-2}\) \(1.02 \times 10^{-9}\) [\(3.3 \times 10^{-13}\)] |
| ácido arsénico | \(\ce{H3AsO4}\) |
2.24 6.96 11.50 |
\(5.8 \times 10^{-3}\) \(1.1 \times 10^{-7}\) \(3.2 \times 10^{-12}\) |
| asparagina (\(\mu = 0.1 \text{ M}\)) | 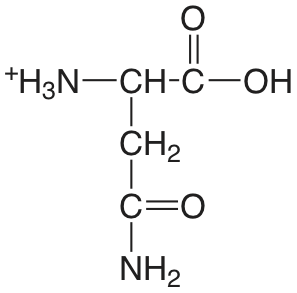 |
2.14 (COOH) 8.72 (\(\ce{NH3}\)) |
\(7.2 \times 10^{-3}\) \(1.9 \times 10^{-9}\) |
| ácido aspártico | 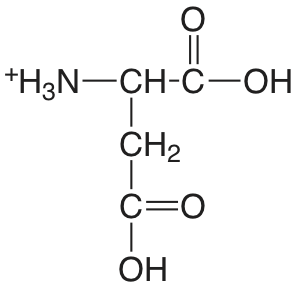 |
1.990 (\(\alpha\)-COOH) 3.900 (\(\beta\)-COOH) 10.002 (\(\ce{NH3}\)) |
\(1.02 \times 10^{-2}\) \(1.26 \times 10^{-4}\) \(9.95 \times 10^{-11}\) |
| ácido benzoico | 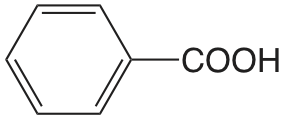 |
4.202 | \(6.28 \times 10^{-5}\) |
| bencilamina | 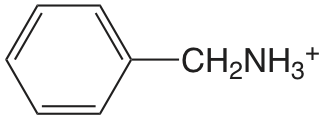 |
9.35 | \(4.5 \times 10^{-10}\) |
| ácido bórico (\(pK_\text{a2}, pK_\text{a3: } T = 20 \text{°C}\)) | \(\ce{H3BO3}\) |
9.236 [12.74] [13.80] |
\(5.81 \times 10^{-10}\) [\(1.82 \times 10^{-13}\)] [\(1.58 \times 10^{-14}\)] |
| ácido carbónico | \(\ce{H2CO3}\) |
6.352 10.329 |
\(4.45 \times 10^{-7}\) \(4.69 \times 10^{-11}\) |
| catecol | 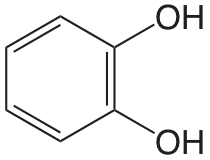 |
9.40 12.8 |
\(4.0 \times 10^{-10}\) \(1.6 \times 10^{-13}\) |
| ácido cloracético | \(\ce{ClCH2COOH}\) | 2.865 | \(1.36 \times 10^{-3}\) |
| ácido crómico (\(pK_\text{a1: } T = 20 \text{°C}\)) | \(\ce{H2CrO4}\) |
—0.2 6.51 |
1.6 \(3.1 \times 10^{-7}\) |
| ácido cítrico | 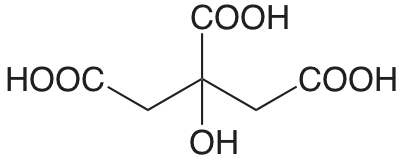 |
3.128 (COOH) 4.761 (COOH) 6.396 (COOH) |
\(7.45 \times 10^{-4}\) \(1.73 \times 10^{-5}\) \(4.02 \times 10^{-7}\) |
| cupferron (\(\mu = 0.1 \text{ M}\)) | 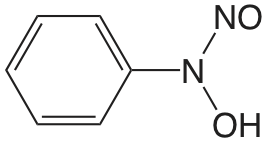 |
4.16 | \(6.9 \times 10^{-5}\) |
| cisteína | 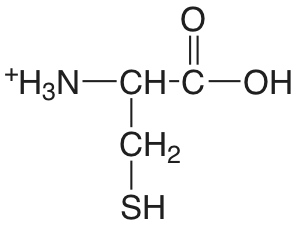 |
[1.71] (COOH) 8.36 (SH) 10.77 (\(\ce{NH3}\)) |
[\(1.9 \times 10^{-2}\)] \(4.4 \times 10^{-9}\) \(1.7 \times 10^{-11}\) |
| ácido dicloracético | \(\ce{Cl2CHCOOH}\) | 1.30 | \(5.0 \times 10^{-2}\) |
| dietilamina | \(\ce{(CH3CH2)2NH2+}\) | 10.933 | \(1.17 \times 10^{-11}\) |
| dimetilamina | \(\ce{(CH3)2NH2+}\) | 10.774 | \(1.68 \times 10^{-11}\) |
| dimetilglioxima | 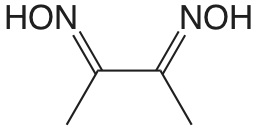 |
10.66 12.0 |
\(2.2 \times 10^{-11}\) \(1. \times 10^{-12}\) |
| etilamina | \(\ce{CH3CH2NH3+}\) | 10.636 | \(2.31 \times 10^{-11}\) |
| etilendiamina | \(\ce{+H3NCH2CH2NH3+}\) |
6.848 9.928 |
\(1.42 \times 10^{-7}\) \(1.18 \times 10^{-10}\) |
|
ácido etilendiaminotetraacético (EDTA) (\(\mu = 0.1 \text{ M}\)) |
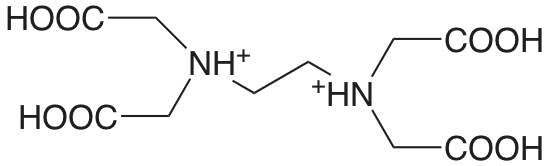 |
0.0 (COOH) 1.5 (COOH) 2.0 (COOH) 2.66 (COOH) 6.16 (NH) 10.24 (NH) |
1.0 \(3.2 \times 10^{-2}\) \(1.0 \times 10^{-2}\) \(2.2 \times 10^{-3}\) \(6.9 \times 10^{-7}\) \(5.8 \times 10^{-11}\) |
| ácido fórmico | \(\ce{HCOOH}\) | 3.745 | \(1.80 \times 10^{-4}\) |
| ácido fumárico | 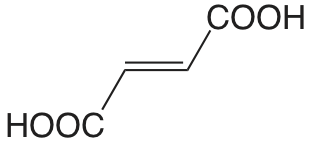 |
3.053 4.494 |
\(8.85 \times 10^{-4}\) \(3.21 \times 10^{-5}\) |
| ácido glutámico | 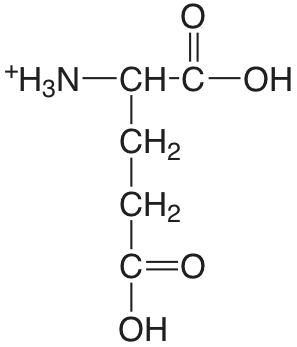 |
2.33 (\(\alpha\)-COOH) 4.42 (\(\lambda\)-COOH) 9.95 (\(\ce{NH3}\)) |
\(5.9 \times 10^{-3}\) \(3.8\times 10^{-5}\) \(1.12 \times 10^{-10}\) |
| glutamina | 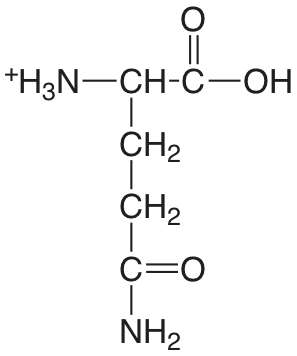 |
2.17 (COOH) 9.01 (\(\ce{NH3}\)) |
\(6.8 \times 10^{-3}\) \(9.8 \times 10^{-10}\) |
| glicina | 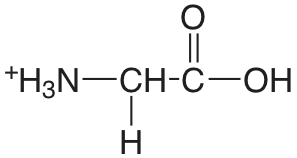 |
2.350 (COOH) 9.778 (\(\ce{NH3}\)) |
\(4.47 \times 10^{-3}\) \(1.67 \times 10^{-10}\) |
| ácido glicólico | \(\ce{HOOCH2COOH}\) |
3.881 (COOH) |
\(1.48 \times 10^{-4}\) |
| histidina (\(\mu = 0.1 \text{ M}\)) | 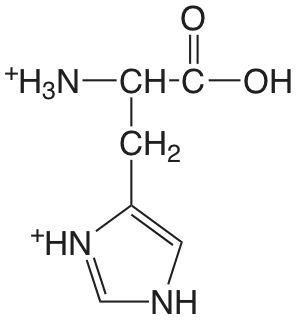 |
1.7 (COOH) 6.02 (NH) 9.08 (\(\ce{NH3}\)) |
\(2. \times 10^{-2}\) \(9.5 \times 10^{-7}\) \(8.3 \times 10^{-10}\) |
| cianuro de hidrógeno | \(\ce{HCN}\) | 9.21 | \(6.2 \times 10^{-10}\) |
| fluoruro de hidrógeno | \(\ce{HF}\) | 3.17 | \(6.8 \times 10^{-4}\) |
| peróxido de hidrógeno | \(\ce{H2O2}\) | 11.65 | \(2.2 \times 10^{-12}\) |
| sulfuro de hidrógeno | \(\ce{H2S}\) |
7.02 13.9 |
\(9.5 \times 10^{-8}\) \(1.3 \times 10^{-14}\) |
| tiocianato de hidrógeno | \(\ce{HSCN}\) | 0.9 | \(1.3 \times 10^{-1}\) |
| 8-hidroxiquinolina | 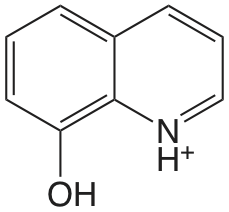 |
4.9 (NH) 9.81 (OH) |
\(1.2 \times 10^{-5}\) \(1.6 \times 10^{-10}\) |
| hidroxilamina | \(\ce{HONH3+}\) | 5.96 | \(1.1 \times 10^{-6}\) |
| ácido hipobromoso | \(\ce{HOBr}\) | 8.63 | \(2.3 \times 10^{-9}\) |
| ácido hipocloroso | \(\ce{HOCl}\) | 7.53 | \(3.0\times 10^{-8}\) |
| ácido hipoyodoso | \(\ce{HOI}\) | 10.64 | \(2.3 \times 10^{-11}\) |
| ácido yódico | \(\ce{HIO3}\) | 0.77 | \(1.7 \times 10^{-1}\) |
| isoleucina | 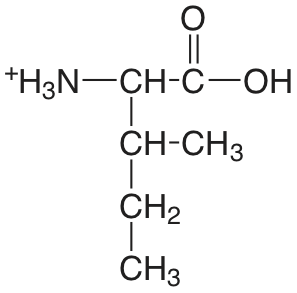 |
2.319 (COOH) 9.754 (\(\ce{NH3}\)) |
\(4.8 \times 10^{-3}\) \(1.76 \times 10^{-10}\) |
| leucina | 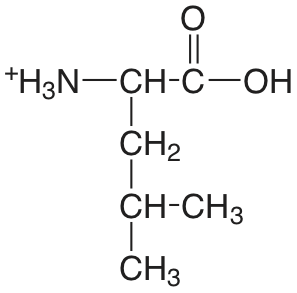 |
2.329 (COOH) 9.747 (\(\ce{NH3}\)) |
\(4.69 \times 10^{-3}\) \(1.79 \times 10^{-10}\) |
| lisina (\(\mu = 0.1 \text{ M}\)) | 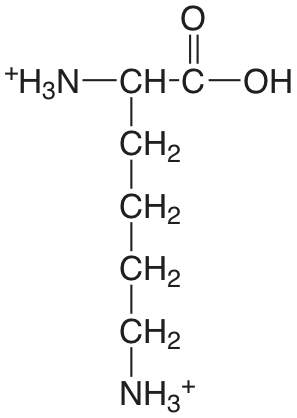 |
2.04 (COOH) 9.08 (\(\alpha \text{-} \ce{NH3}\)) 10.69 (\(\epsilon \text{-} \ce{NH3}\)) |
\(9.1 \times 10^{-3}\) \(8.3 \times 10^{-10}\) \(2.0 \times 10^{-11}\) |
| ácido maleico | 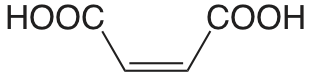 |
1.910 6.332 |
\(1.23 \times 10^{-2}\) \(4.66 \times 10^{-7}\) |
| ácido málico | 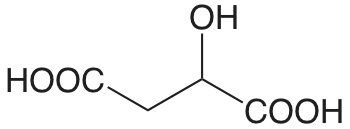 |
3.459 (COOH) 5.097 (COOH) |
\(3.48 \times 10^{-4}\) \(8.00 \times 10^{-6}\) |
| ácido malónico | \(\ce{HOOCCH2COOH}\) |
2.847 5.696 |
\(1.42 \times 10^{-3}\) \(2.01 \times 10^{-6}\) |
| metionina (\(\mu = 0.1 \text{ M}\)) | 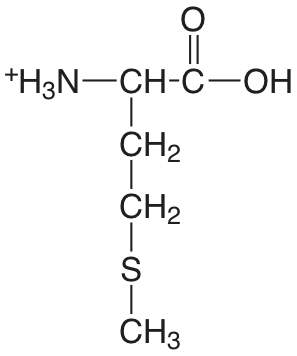 |
2.20 (COOH) 9.05 (\(\ce{NH3}\)) |
\(6.3 \times 10^{-3}\) \(8.9 \times 10^{-10}\) |
| metilamina | \(\ce{CH3NH3+}\) | 10.64 | \(2.3 \times 10^{-11}\) |
| 2-metilanalina | 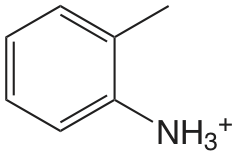 |
4.447 | \(3.57 \times 10^{-5}\) |
| 4-metilanalina | 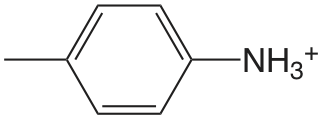 |
5.084 | \(8.24 \times 10^{-6}\) |
| 2-metilfenol | 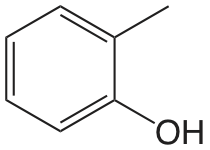 |
10.28 | \(5.2 \times 10^{-11}\) |
| 4-metilfenol | 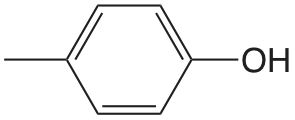 |
10.26 | \(5.5 \times 10^{-11}\) |
|
ácido nitrilotriacético (\(T = 20 \text{°C}), pK_\text{a1: } \mu = 0.1 \text{ M}\)) |
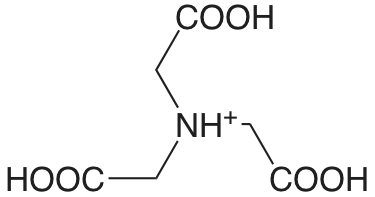 |
1.1 (COOH) 1.650 (COOH) 2.940 (COOH) 10.334 (\(\ce{NH3}\)) |
\(8. \times 10^{-2}\) \(2.24 \times 10^{-2}\) \(1.15 \times 10^{-3}\) \(4.63 \times 10^{-11}\) |
| Ácido 2-nitrobenzoico | 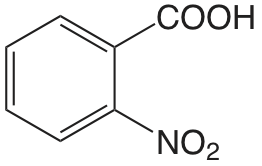 |
2.179 | \(6.62 \times 10^{-3}\) |
| Ácido 3-nitrobenzoico | 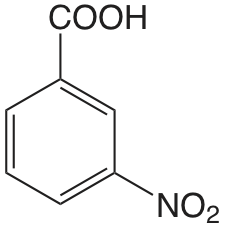 |
3.449 | \(3.56 \times 10^{-4}\) |
| Ácido 4-nitrobenzoico | 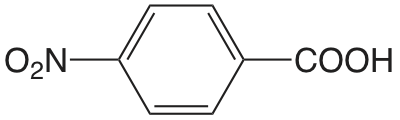 |
3.442 | \(3.61 \times 10^{-4}\) |
| 2-nitrofenol | 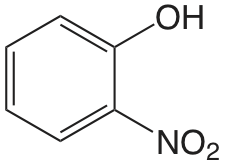 |
7.21 | \(6.2 \times 10^{-8}\) |
| 3-nitrofenol | 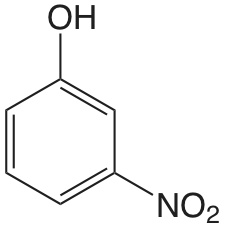 |
8.39 | \(4.1 \times 10^{-9}\) |
| 4-nitrofenol | 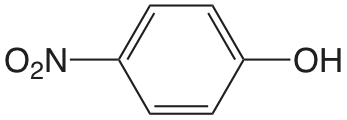 |
7.15 | \(7.1 \times 10^{-8}\) |
| ácido nitroso | \(\ce{HNO2}\) | 3.15 | \(7.1 \times 10^{-4}\) |
| ácido oxálico | \(\ce{H2C2O4}\) |
1.252 4.266 |
\(5.60 \times 10^{-2}\) \(5.42 \times 10^{-5}\) |
| 1,10-fenantrolina | 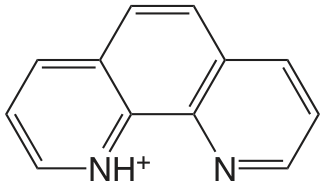 |
4.86 | \(1.38 \times 10^{-5}\) |
| fenol | 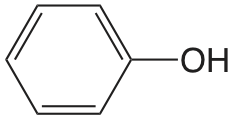 |
9.98 | \(1.05 \times 10^{-10}\) |
| fenilalanina | 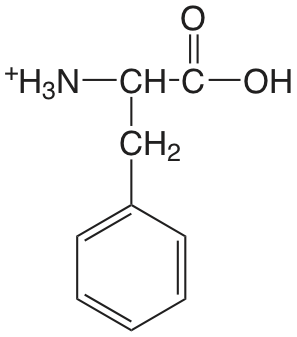 |
2.20 (COOH) 9.31 (\(\ce{NH3}\)) |
\(6.3 \times 10^{-3}\) \(4.9 \times 10^{-10}\) |
| ácido fosfórico | \(\ce{H3PO4}\) |
2.148 7.199 12.35 |
\(7.11 \times 10^{-3}\) \(6.32 \times 10^{-8}\) \(4.5 \times 10^{-13}\) |
| ácido ftálico | 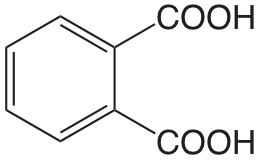 |
2.950 5.408 |
\(1.12 \times 10^{-3}\) \(3.91 \times 10^{-6}\) |
| piperdina | 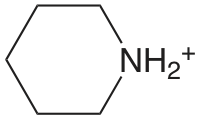 |
11.123 | \(7.53 \times 10^{-12}\) |
| prolina | 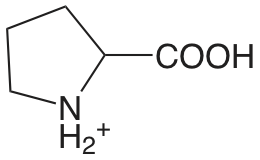 |
1.952 (COOH) 10.650 (NH) |
\(1.12 \times 10^{-2}\) \(2.29 \times 10^{-11}\) |
| ácido propanoico | \(\ce{CH3CH2COOH}\) |
4.874 |
\(1.34 \times 10^{-5}\) |
| propilamina | \(\ce{CH3CH2CH2NH3+}\) | 10.566 | \(2.72 \times 10^{-11}\) |
| piridina | 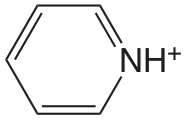 |
5.229 | \(5.90 \times 10^{-6}\) |
| resorcinol | 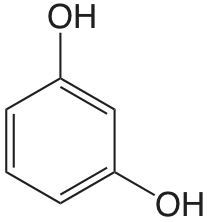 |
9.30 11.06 |
\(5.0 \times 10^{-10}\) \(8.7 \times 10^{-12}\) |
| ácido salicílico | 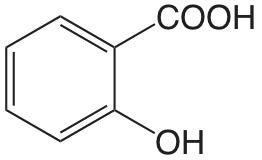 |
2.97 (COOH) 13.74 (OH) |
\(1.1 \times 10^{-3}\) \(1.8 \times 10^{-14}\) |
| serina | 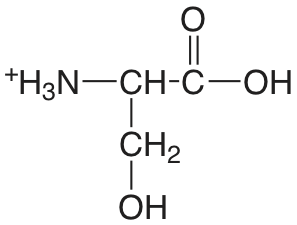 |
2.187 (COOH) 9.209 (\(\ce{NH3}\)) |
\(6.50 \times 10^{-3}\) \(6.18 \times 10^{-10}\) |
| ácido succínico | 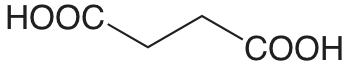 |
4.207 5.636 |
\(6.21 \times 10^{-5}\) \(2.31 \times 10^{-6}\) |
| ácido sulfúrico | \(\ce{H2SO4}\) |
fuerte 1.99 |
— \(1.0 \times 10^{-2}\) |
| ácido sulfuroso | \(\ce{H2SO3}\) |
1.91 7.18 |
\(1.2 \times 10^{-2}\) \(6.6 \times 10^{-8}\) |
| Ácido D-tartárico | 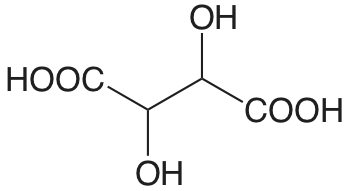 |
3.036 (COOH) 4.366 (COOH) |
\(9.20 \times 10^{-4}\) \(4.31 \times 10^{-5}\) |
| treonina | 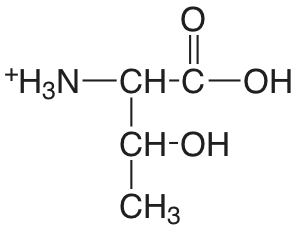 |
2.088 (COOH) 9.100 (\(\ce{NH3}\)) |
\(8.17 \times 10^{-3}\) \(7.94 \times 10^{-10}\) |
| ácido tiosulfúrico | \(\ce{H2S2O3}\) |
0.6 1.6 |
\(3. \times 10^{-1}\) \(3. \times 10^{-2}\) |
| ácido tricloracético (\(\mu = 0.1 \text{ M}\)) | \(\ce{Cl3CCOOH}\) | 0.66 | \(2.2 \times 10^{-1}\) |
| trietanolamina | \(\ce{(HOCH2CH2)3NH+}\) | 7.762 | \(1.73 \times 10^{-8}\) |
| trietilamina | \(\ce{(CH3CH2)3NH+}\) | 10.715 | \(1.93 \times 10^{-11}\) |
| trimetilamina | \(\ce{(CH3)3NH+}\) | 9.800 | \(1.58 \times 10^{-10}\) |
| tris (hidroximetil) amino metano (TRIS o THAM) | \(\ce{(HOCH2)3CNH3+}\) | 8.075 | \(8.41 \times 10^{-9}\) |
| triptófano (\(\mu = 0.1 \text{ M}\)) | 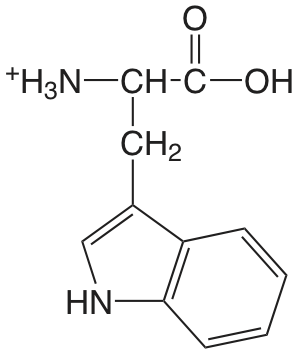 |
2.35 (COOH) 9.33 (\(\ce{NH3}\)) |
\(4.5 \times 10^{-3}\) \(4.7 \times 10^{-10}\) |
| tirosina (\(pK_\text{a1: } \mu = 0.1 \text{ M}\)) | 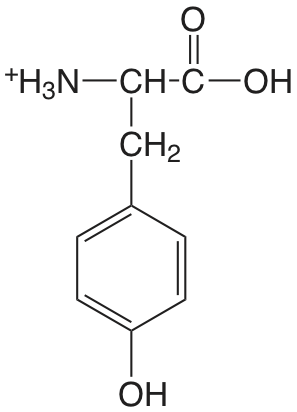 |
2.17 (COOH) 9.19 (\(\ce{NH3}\)) 10.47 (OH) |
\(6.8 \times 10^{-3}\) \(6.5 \times 10^{-10}\) \(3.4 \times 10^{-11}\) |
| valina | 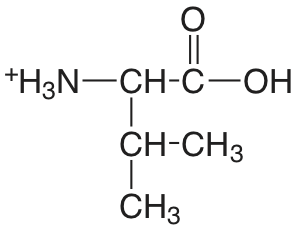 |
2.286 (COOH) 9.718 (\(\ce{NH3}\)) |
\(5.18 \times 10^{-3}\) \(1.91 \times 10^{-10}\) |

