16.12: Constantes de Formación
- Page ID
- 75460
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)La siguiente tabla proporciona\(K_i\) y\(\beta_i\) valores para los complejos metal-ligando seleccionados, dispuestos por el ligando. Todos los valores son de Martell, A. E.; Smith, R. M. Constantes de Estabilidad Crítica, Vols. 1—4. Plenum Press: Nueva York, 1976. A menos que se indique lo contrario, los valores son para 25 o C y fuerza iónica cero. Esos valores entre paréntesis se consideran menos confiables.
|
Acetato \(\ce{CH3COO-}\) |
registro\(K_1\) | registro\(K_2\) | registro\(K_3\) | registro\(K_4\) | registro\(K_5\) | registro\(K_6\) |
|---|---|---|---|---|---|---|
| \ (\ ce {CH3COO-}\) ">Mg 2 + | \ (K_1\) ">1.27 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CH3COO-}\) ">Ca 2+ | \ (K_1\) ">1.18 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CH3COO-}\) ">Ba 2 + | \ (K_1\) ">1.07 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CH3COO-}\) ">Mn 2 + | \ (K_1\) ">1.40 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CH3COO-}\) ">Fe 2+ | \ (K_1\) ">1.40 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CH3COO-}\) ">Co 2 + | \ (K_1\) ">1.46 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CH3COO-}\) ">Ni 2+ | \ (K_1\) ">1.43 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CH3COO-}\) ">Cu 2 + | \ (K_1\) ">2.22 | \ (K_2\) ">1.41 | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CH3COO-}\) ">Ag + | \ (K_1\) ">0.73 | \ (K_2\) ">—0.09 | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CH3COO-}\) ">Zn 2+ | \ (K_1\) ">1.57 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CH3COO-}\) ">Cd 2 + | \ (K_1\) ">1.93 | \ (K_2\) ">1.22 | \ (K_3\) ">—0.89 | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CH3COO-}\) ">Pb 2+ | \ (K_1\) ">2.68 | \ (K_2\) ">1.40 | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
|
Amoníaco \(\ce{NH3}\) |
registro\(K_1\) | registro\(K_2\) | registro\(K_3\) | registro\(K_4\) | registro\(K_5\) | registro\(K_6\) |
|---|---|---|---|---|---|---|
| \ (\ ce {NH3}\) ">Ag + | \ (K_1\) ">3.31 | \ (K_2\) ">3.91 | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {NH3}\) ">Co 2+ (T = 20 °C) | \ (K_1\) ">1.99 | \ (K_2\) ">1.51 | \ (K_3\) ">0.93 | \ (K_4\) ">0.64 | \ (K_5\) ">0.06 | \ (K_6\) ">—0.73 |
| \ (\ ce {NH3}\) ">Ni 2 + | \ (K_1\) ">2.72 | \ (K_2\) ">2.17 | \ (K_3\) ">1.66 | \ (K_4\) ">1.12 | \ (K_5\) ">0.67 | \ (K_6\) ">—0.03 |
| \ (\ ce {NH3}\) ">Cu 2+ | \ (K_1\) ">4.04 | \ (K_2\) ">3.43 | \ (K_3\) ">2.80 | \ (K_4\) ">1.48 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {NH3}\) ">Zn 2 + | \ (K_1\) ">2.21 | \ (K_2\) ">2.29 | \ (K_3\) ">2.36 | \ (K_4\) ">2.03 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {NH3}\) ">Cd 2+ | \ (K_1\) ">2.55 | \ (K_2\) ">2.01 | \ (K_3\) ">1.34 | \ (K_4\) ">0.84 | \ (K_5\) "> | \ (K_6\) "> |
|
Cloruro \(\ce{Cl-}\) |
registro\(K_1\) | registro\(K_2\) | registro\(K_3\) | registro\(K_4\) | registro\(K_5\) | registro\(K_6\) |
|---|---|---|---|---|---|---|
| \ (\ ce {Cl-}\) ">Cu 2 + | \ (K_1\) ">0.40 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {Cl-}\) ">Fe 3+ | \ (K_1\) ">1.48 | \ (K_2\) ">0.65 | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {Cl-}\)” class="lt-chem-135872">
Ag + (\(\mu = 5.0 \text{ M}\)) |
\ (K_1\) ">3.70 | \ (K_2\) ">1.92 | \ (K_3\) ">0.78 | \ (K_4\) ">—0.3 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {Cl-}\) ">Zn 2 + | \ (K_1\) ">0.43 | \ (K_2\) ">0.18 | \ (K_3\) ">—0.11 | \ (K_4\) ">—0.3 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {Cl-}\) ">Cd 2+ | \ (K_1\) ">1.98 | \ (K_2\) ">1.62 | \ (K_3\) ">—0.2 | \ (K_4\) ">—0.7 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {Cl-}\) ">Pb 2 + | \ (K_1\) ">1.59 | \ (K_2\) ">0.21 | \ (K_3\) ">—0.1 | \ (K_4\) ">—0.3 | \ (K_5\) "> | \ (K_6\) "> |
|
Cianuro \(\ce{CN-}\) |
registro\(K_1\) | registro\(K_2\) | registro\(K_3\) | registro\(K_4\) | registro\(K_5\) | registro\(K_6\) |
|---|---|---|---|---|---|---|
| \ (\ ce {CN-}\) ">Fe 2 + | \ (K_1\) "> | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) ">35.4 (\(\beta_6\)) |
| \ (\ ce {CN-}\) ">Fe 3+ | \ (K_1\) "> | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) ">43.6 (\(\beta_6\)) |
| \ (\ ce {CN-}\) ">Ag + | \ (K_1\) "> | \ (K_2\) ">20.48 (\(\beta_2\)) | \ (K_3\) ">0.92 | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CN-}\) ">Zn 2+ | \ (K_1\) "> | \ (K_2\) ">11.07 (\(\beta_2\)) | \ (K_3\) ">4.98 | \ (K_4\) ">3.57 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CN-}\) ">Cd 2 + | \ (K_1\) ">6.01 | \ (K_2\) ">5.11 | \ (K_3\) ">4.53 | \ (K_4\) ">2.27 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CN-}\) ">Hg 2+ | \ (K_1\) ">17.00 | \ (K_2\) ">15.75 | \ (K_3\) ">3.56 | \ (K_4\) ">2.66 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {CN-}\) ">Ni 2 + | \ (K_1\) "> | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) ">30.22 (\(\beta_4\)) | \ (K_5\) "> | \ (K_6\) "> |
|
Etilendiamina \(\ce{H2NCH2CH2NH2}\) |
registro\(K_1\) | registro\(K_2\) | registro\(K_3\) | registro\(K_4\) | registro\(K_5\) | registro\(K_6\) |
|---|---|---|---|---|---|---|
| \ (\ ce {H2NCH2CH2NH2}\) ">Ni 2 + | \ (K_1\) ">7.38 | \ (K_2\) ">6.18 | \ (K_3\) ">4.11 | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {H2NCH2CH2NH2}\) ">Cu 2+ | \ (K_1\) ">10.48 | \ (K_2\) ">9.07 | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {H2NCH2CH2NH2}\) ">Ag + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">4.700 | \ (K_2\) ">3.00 | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {H2NCH2CH2NH2}\) ">Zn 2 + | \ (K_1\) ">5.66 | \ (K_2\) ">4.98 | \ (K_3\) ">3.25 | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {H2NCH2CH2NH2}\) ">Cd 2+ | \ (K_1\) ">5.41 | \ (K_2\) ">4.50 | \ (K_3\) ">2.78 | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
|
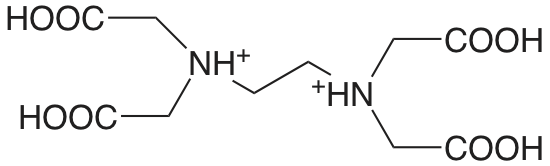
EDTA
|
registro\(K_1\) | registro\(K_2\) | registro\(K_3\) | registro\(K_4\) | registro\(K_5\) | registro\(K_6\) |
|---|---|---|---|---|---|---|
| Mg 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">8.79 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Ca 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">10.69 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Ba 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">7.86 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Bi 3 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">27.8 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Co 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">16.31 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Ni 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">18.62 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Cu 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">18.80 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Cr 3 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) "> [23.4] | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Fe 3 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">25.1 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Ag + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">7.32 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Zn 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">16.50 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Cd 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">16.46 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Hg 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">21.7 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Pb 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">18.04 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Al 3 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">16.3 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
|
Fluoruro \(\ce{F-}\) |
registro\(K_1\) | registro\(K_2\) | registro\(K_3\) | registro\(K_4\) | registro\(K_5\) | registro\(K_6\) |
|---|---|---|---|---|---|---|
| \ (\ ce {F-}\) ">Al 3 + | \ (K_1\) ">6.11 | \ (K_2\) ">5.01 | \ (K_3\) ">3.88 | \ (K_4\) ">3.0 | \ (K_5\) ">1.4 | \ (K_6\) ">0.4 |
|
Hidróxido \(\ce{OH-}\) |
registro\(K_1\) | registro\(K_2\) | registro\(K_3\) | registro\(K_4\) | registro\(K_5\) | registro\(K_6\) |
|---|---|---|---|---|---|---|
| \ (\ ce {OH-}\) ">Al 3 + | \ (K_1\) ">9.01 | \ (K_2\) "> [9.69] | \ (K_3\) "> [8.3] | \ (K_4\) ">6.0 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {OH-}\) ">Co 2 + | \ (K_1\) ">4.3 | \ (K_2\) ">4.1 | \ (K_3\) ">1.3 | \ (K_4\) ">0.5 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {OH-}\) ">Fe 2+ | \ (K_1\) ">4.5 | \ (K_2\) ">] 2.9] | \ (K_3\) ">2,6 | \ (K_4\) ">—0.4 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {OH-}\) ">Fe 3 + | \ (K_1\) ">11.81 | \ (K_2\) ">10.5 | \ (K_3\) ">12.1 | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {OH-}\) ">Ni 2+ | \ (K_1\) ">4.1 | \ (K_2\) ">3.9 | \ (K_3\) ">3. | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {OH-}\) ">Pb 2 + | \ (K_1\) ">6.3 | \ (K_2\) ">4.6 | \ (K_3\) ">3.0 | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {OH-}\) ">Zn 2+ | \ (K_1\) ">5.0 | \ (K_2\) "> [6.1] | \ (K_3\) ">2.5 | \ (K_4\) "> [1.2] | \ (K_5\) "> | \ (K_6\) "> |
|
yoduro \(\ce{I-}\) |
registro\(K_1\) | registro\(K_2\) | registro\(K_3\) | registro\(K_4\) | registro\(K_5\) | registro\(K_6\) |
|---|---|---|---|---|---|---|
| \ (\ ce {I-}\) ">Ag + | \ (K_1\) ">6.58 | \ (K_2\) "> [5.12] | \ (K_3\) "> [1.4] | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {I-}\) ">Cd 2+ (T = 18 °C) | \ (K_1\) ">2.28 | \ (K_2\) ">1.64 | \ (K_3\) ">1.08 | \ (K_4\) ">1.0 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {I-}\) ">Pb 2 + | \ (K_1\) ">1.92 | \ (K_2\) ">1.28 | \ (K_3\) ">0.7 | \ (K_4\) ">0.6 | \ (K_5\) "> | \ (K_6\) "> |
|
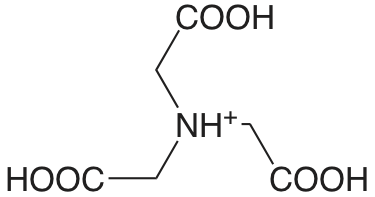
Nitriloacetato
|
registro\(K_1\) | registro\(K_2\) | registro\(K_3\) | registro\(K_4\) | registro\(K_5\) | registro\(K_6\) |
|---|---|---|---|---|---|---|
| Mg 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">5.41 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Ca 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">6.41 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Ba 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">4.82 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Mn 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">7.44 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Fe 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">8.33 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Co 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">10.38 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Ni 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">11.53 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Cu 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">12.96 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Fe 3 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">15.9 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Zn 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">10.67 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Cd 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">9.83 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Pb 2 + (T = 20 °C,\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">11.39 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
|
Oxalato \(\ce{C2O4^{2-}}\) |
registro\(K_1\) | registro\(K_2\) | registro\(K_3\) | registro\(K_4\) | registro\(K_5\) | registro\(K_6\) |
|---|---|---|---|---|---|---|
| \ (\ ce {C2O4^ {2-}}\) ">Ca 2 + (\(\mu = 1 \text{ M}\)) | \ (K_1\) ">1.66 | \ (K_2\) ">1.03 | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {C2O4^ {2-}}\) ">Fe 2 + (\(\mu = 1 \text{ M}\)) | \ (K_1\) ">3.05 | \ (K_2\) ">2.10 | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {C2O4^ {2-}}\) ">Co 2 + | \ (K_1\) ">4.72 | \ (K_2\) ">2.28 | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {C2O4^ {2-}}\) ">Ni 2 + | \ (K_1\) ">5.16 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {C2O4^ {2-}}\) ">Cu 2 + | \ (K_1\) ">6.23 | \ (K_2\) ">4.04 | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {C2O4^ {2-}}\) ">Fe 3 + (\(\mu = 0.5 \text{ M}\)) | \ (K_1\) ">7.53 | \ (K_2\) ">6.11 | \ (K_3\) ">4.83 | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {C2O4^ {2-}}\) ">Zn 2 + | \ (K_1\) ">4.87 | \ (K_2\) ">2.78 | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
|
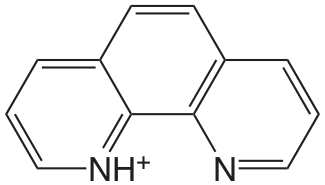
1,10-fenantrolina
|
registro\(K_1\) | registro\(K_2\) | registro\(K_3\) | registro\(K_4\) | registro\(K_5\) | registro\(K_6\) |
|---|---|---|---|---|---|---|
| Fe 2 + | \ (K_1\) "> | \ (K_2\) "> | \ (K_3\) ">20.7 (\(\beta_3\)) | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Mn 2 + (\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">4.0 | \ (K_2\) ">3.3 | \ (K_3\) ">3.0 | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Cu 2 + (\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">7.08 | \ (K_2\) ">6.64 | \ (K_3\) ">6.08 | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Ni 2 + | \ (K_1\) ">8.6 | \ (K_2\) ">8.1 | \ (K_3\) ">7.6 | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Fe 3 + | \ (K_1\) "> | \ (K_2\) "> | \ (K_3\) ">13.8 (\(\beta_3\)) | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Ag + (\(\mu = 0.1 \text{ M}\)) | \ (K_1\) ">5.02 | \ (K_2\) ">7.04 | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| Zn 2 + | \ (K_1\) ">6.2 | \ (K_2\) "> [5.9] | \ (K_3\) "> [5.2] | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
|
Tiosulfato \(\ce{S2O3^{2-}}\) |
registro\(K_1\) | registro\(K_2\) | registro\(K_3\) | registro\(K_4\) | registro\(K_5\) | registro\(K_6\) |
|---|---|---|---|---|---|---|
| \ (\ ce {S2O3^ {2-}}\) ">Ag + (T = 20 °C) | \ (K_1\) ">8.82 | \ (K_2\) ">4.85 | \ (K_3\) ">0.53 | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
|
Tiocianato \(\ce{SCN-}\) |
registro\(K_1\) | registro\(K_2\) | registro\(K_3\) | registro\(K_4\) | registro\(K_5\) | registro\(K_6\) |
|---|---|---|---|---|---|---|
| \ (\ ce {SCN-}\) ">Mn 2 + | \ (K_1\) ">1.23 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {SCN-}\) ">Fe 2 + | \ (K_1\) ">1.31 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {SCN-}\) ">Co 2 + | \ (K_1\) ">1.71 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {SCN-}\) ">Ni 2 + | \ (K_1\) ">1.76 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {SCN-}\) ">Cu 2 + | \ (K_1\) ">2.33 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {SCN-}\) ">Fe 3 + | \ (K_1\) ">3.02 | \ (K_2\) "> | \ (K_3\) "> | \ (K_4\) "> | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {SCN-}\) ">Ag + | \ (K_1\) ">4.8 | \ (K_2\) ">3.43 | \ (K_3\) ">1.27 | \ (K_4\) ">0.2 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {SCN-}\) ">Zn 2 + | \ (K_1\) ">1.33 | \ (K_2\) ">0.58 | \ (K_3\) ">0.09 | \ (K_4\) ">—0.4 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {SCN-}\) ">Cd 2 + | \ (K_1\) ">1.89 | \ (K_2\) ">0.89 | \ (K_3\) ">0.02 | \ (K_4\) ">—0.5 | \ (K_5\) "> | \ (K_6\) "> |
| \ (\ ce {SCN-}\) ">Hg 2 + | \ (K_1\) "> | \ (K_2\) ">17.26 (\(\beta_2\)) | \ (K_3\) ">2.71 | \ (K_4\) ">1.83 | \ (K_5\) "> | \ (K_6\) "> |