6.4: Diastereómeros - más de un centro quiral
- Page ID
- 76425
Objetivo de aprendizaje
- reconocer y clasificar diastereómeros
Los diastereómeros son estereoisómeros con dos o más centros quirales que no son enantiómeros. Los diastereómeros tienen diferentes propiedades físicas (puntos de fusión, puntos de ebullición y densidades). Dependiendo del mecanismo de reacción, los diastereómeros pueden producir diferentes productos estereoquímicos.
Introducción
Hasta el momento, hemos estado analizando compuestos con un solo centro quiral. A continuación, dirigimos nuestra atención a aquellos que tienen múltiples centros quirales. Comenzaremos con algunos azúcares estereoisoméricos de cuatro carbonos con dos centros quirales.
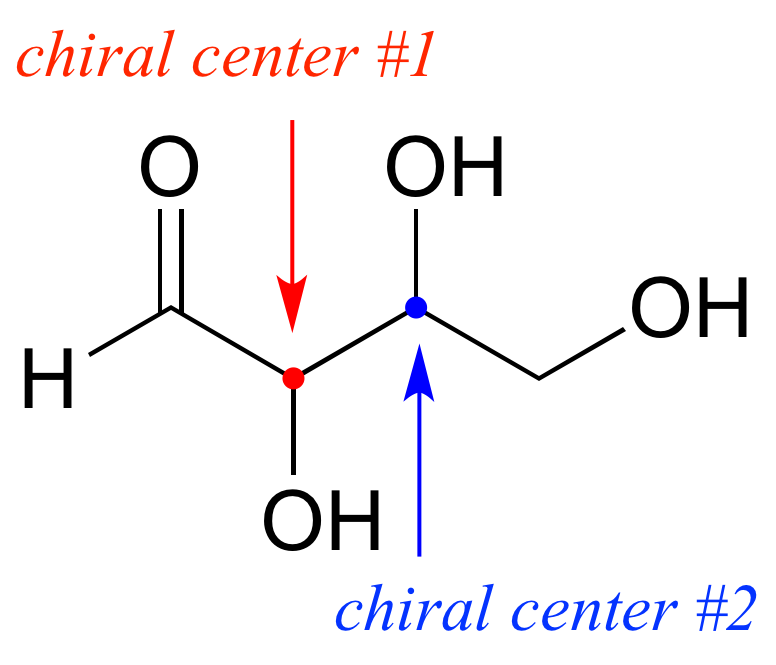
Para evitar confusiones, simplemente nos referiremos a los diferentes estereoisómeros por letras mayúsculas.
Mire primero el compuesto A a continuación. Ambos centros quirales en tienen la configuración R (¡debes confirmarlo tú mismo!). La imagen especular del Compuesto A es el compuesto B, que tiene la configuración S en ambos centros quirales. Si tuviéramos que recoger el compuesto A, darle la vuelta y ponerlo junto al compuesto B, veríamos que no son superponibles (de nuevo, ¡confirme esto usted mismo con sus modelos!). A y B son imágenes especulares no superponibles: en otras palabras, enantiómeros.
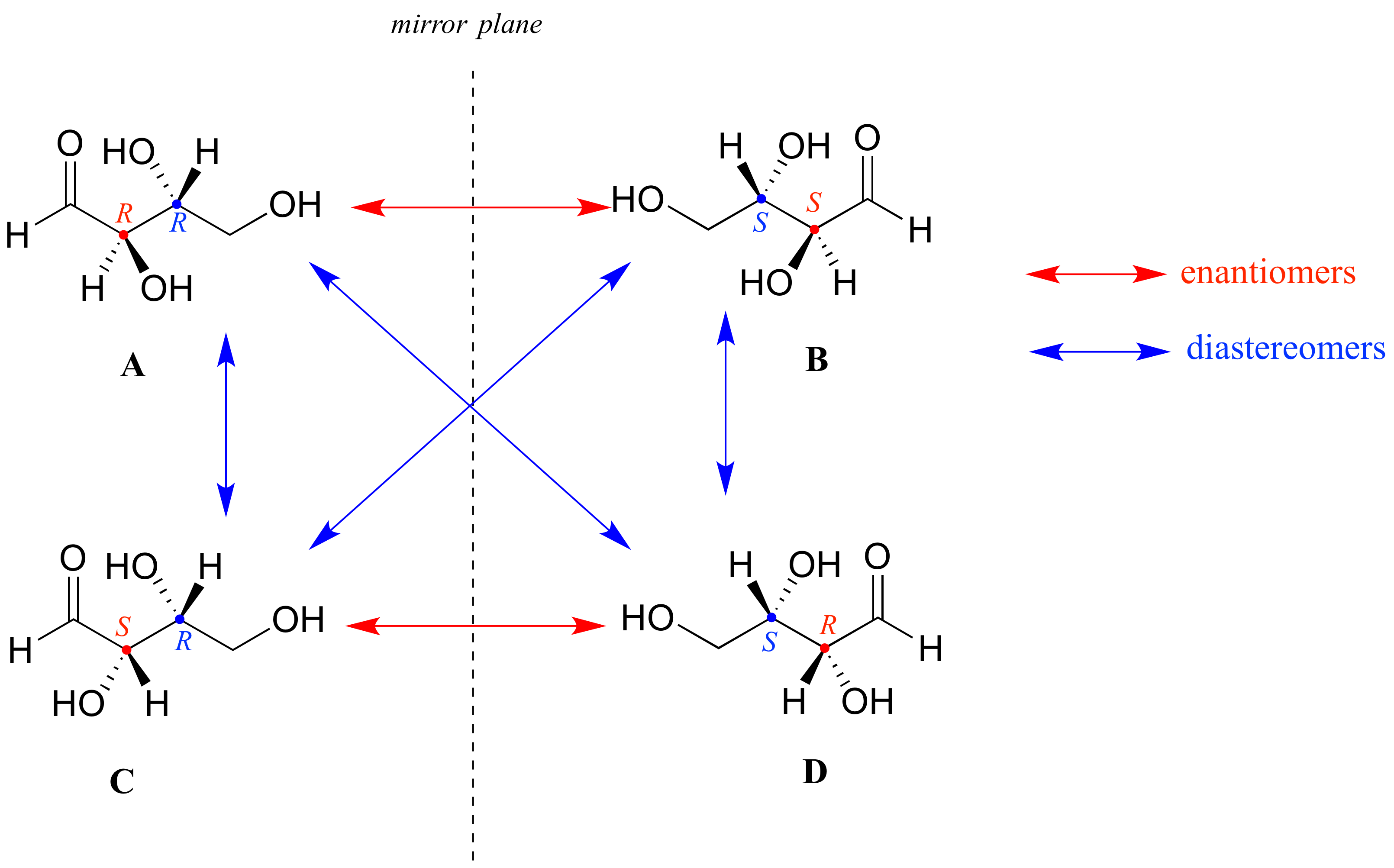
Ahora, mire el compuesto C, en el que la configuración es S en el centro quiral 1 y R en el centro quiral 2. Los compuestos A y C son estereoisómeros: tienen la misma fórmula molecular y la misma conectividad de enlace, pero una disposición diferente de átomos en el espacio (recordemos que esta es la definición del término 'estereoisómero). Sin embargo, no son imágenes especulares entre sí (¡confirma esto con tus modelos!) , y así no son enantiómeros. Por definición, son diastereómeros el uno del otro.
Observe que los compuestos C y B también tienen una relación diastereomérica, por la misma definición.
Entonces, los compuestos A y B son un par de enantiómeros, y el compuesto C es un diastereómero de ambos. ¿El compuesto C tiene su propio enantiómero? El compuesto D es la imagen especular del compuesto C, y los dos no son superponibles. Por lo tanto, C y D son un par de enantiómeros. El compuesto D es también un diastereómero de los compuestos A y B.
Esto también puede parecer muy confuso al principio, pero hay algunos atajos simples para analizar estereoisómeros:

